24-25 Muscle Physiology
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
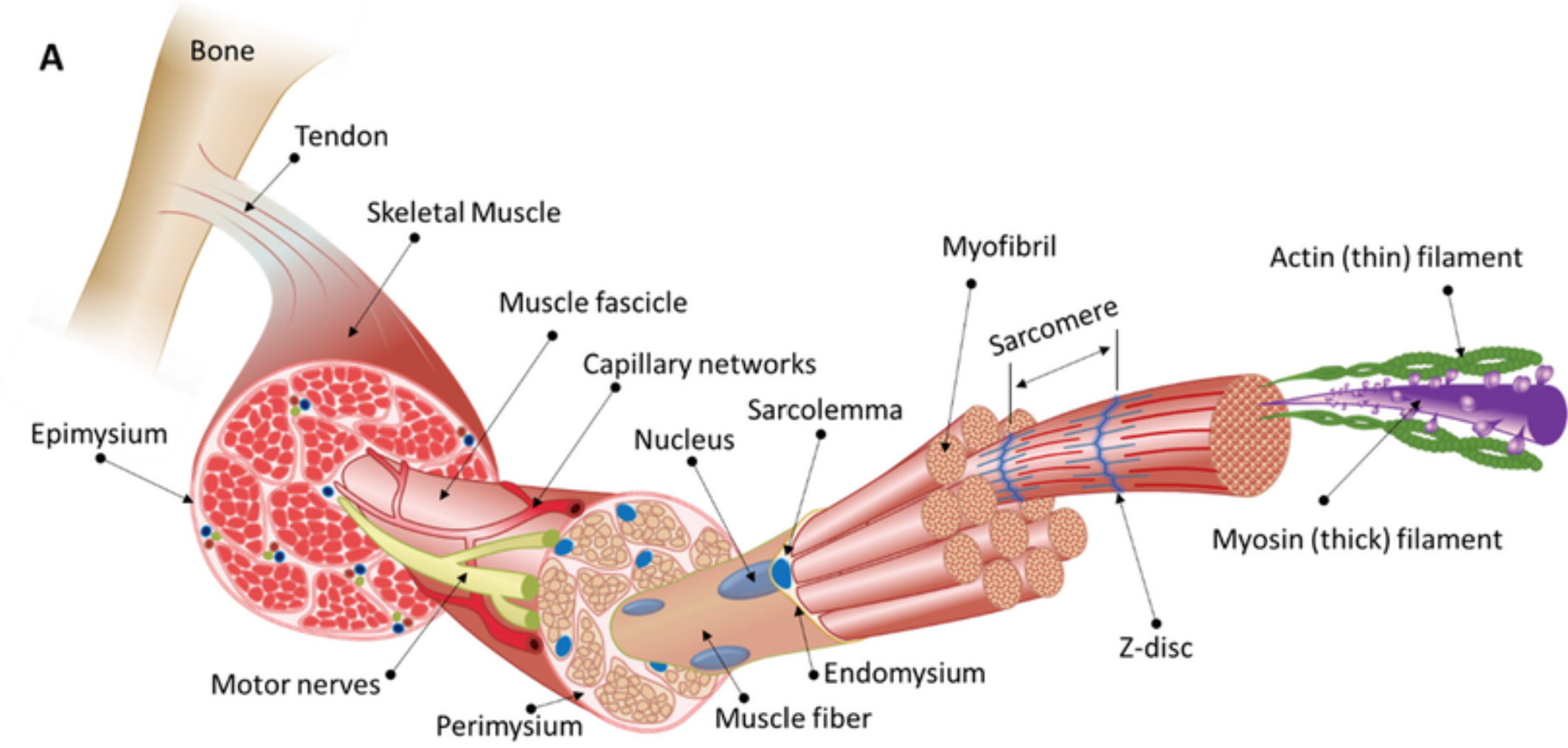
Functional Morphology of Skeletal Muscle
Muscle → Fascicles (bundles) → Muscle Fiber (cell) → Myofibrils → Myofilaments (actin/myosin).
Muscles are composed of fascicles (bundles of muscle fibers).
Muscle Fiber (Cell): Long, cylindrical cell with multiple nuclei. Contains:
Myofibrils: Thread-like structures packed with actin (thin filaments) and myosin (thick filaments)
Sarcolemma connects to tendons
Sarcoplasmic Reticulum (SR): Calcium storage network surrounding myofibrils.
T-Tubules: Invaginations of the sarcolemma that transmit action potentials to the SR.
Supportive layers of connective tissue surround muscle fibres
Endomysium – surrounds individual muscle fibres
Perimysium – surrounds a bundle of muscle fibres forming a fascicle (functional unit)
Epimysium – surrounds the entire muscle
Sarcomere: Functional unit of contraction, bounded by Z-discs (anchor actin). Contains:
A-band: Dark region with overlapping actin and myosin.
I-band: Light region with actin only.
H-zone: Central region of A-band with myosin only (shortens during contraction).
Titin: Giant elastic protein connecting myosin to Z-discs, maintaining sarcomere structure.
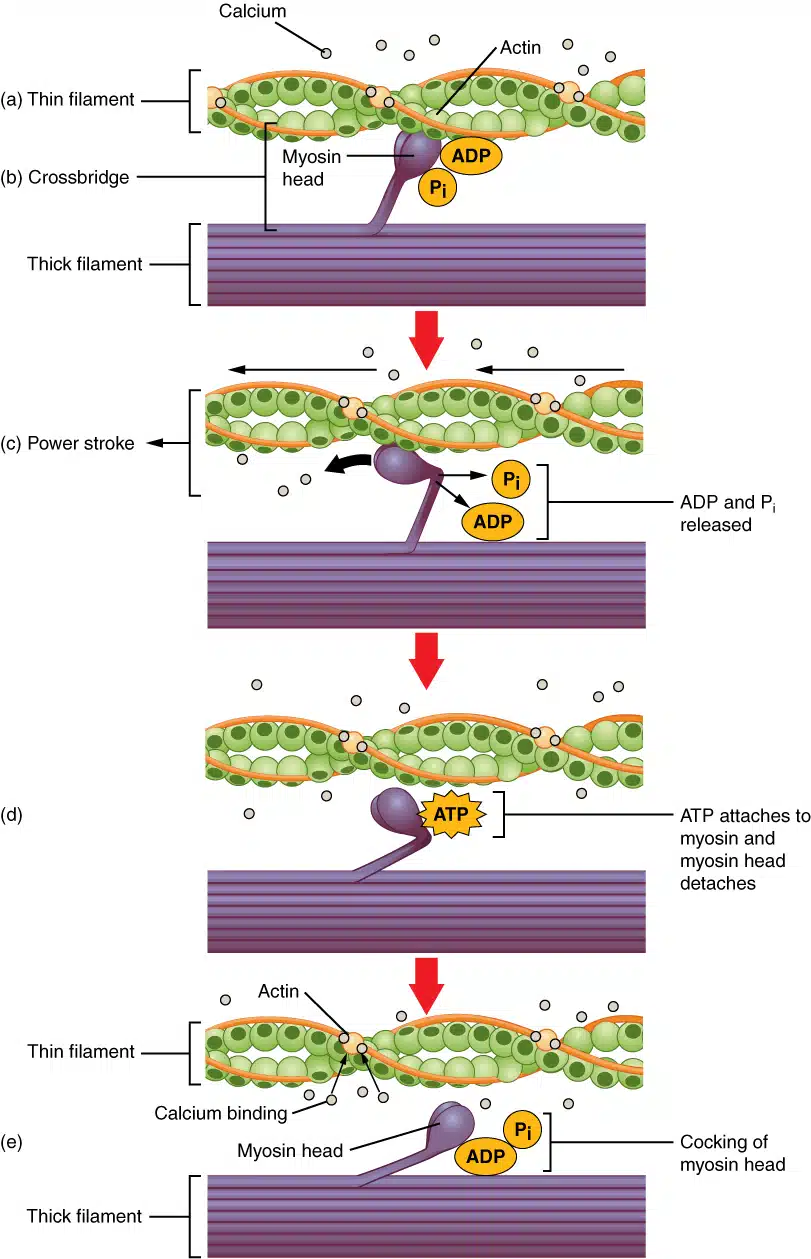
Mechanism of Contraction of Skeletal Muscle: Sliding Filament Theory
Excitation-Contraction Coupling:
Motor neuron releases ACh, triggering muscle membrane depolarization
AP generated at the neuromuscular junction travel along the sarcolemma and down into the transverse tubule (T-tubule) system to depolarise the cell membrane
Depolarisation of the sarcolemma → opening of voltage-gated L-type Ca2+ channels (dihydropyridine receptors), allowing Ca2+ to enter the cell
Ca2+ influx leads to activation of ryanodine receptors located in the SR, which allows Ca2+ to flow from the SR into the cytoplasm and further increases intracellular Ca2+ concentration
Ca2+ binds to troponin-c, inducing a conformational change which exposes a binding site on actin for the myosin head
Results in ATP hydrolysis, providing energy for the actin and myosin filaments to slide past each other and shorten the sarcomere length, thereby initiating muscle contraction
Cross-Bridge Cycling:
Attachment: Myosin heads (energized by ATP hydrolysis) bind actin.
Power Stroke: Myosin heads pivot, pulling actin toward sarcomere center (ADP + Pi released).
Detachment: ATP binds myosin → head releases actin.
Reset: ATP split to ADP + Pi by myosin ATPase → head repositions for next cycle.
"Walk-Along" Mechanism: Repeated cycles cause filaments to slide, shortening sarcomeres (I-bands narrow; H-zone disappears).
Relaxation:
Ca²⁺ Reuptake: SR’s Ca²⁺-ATPase pumps Ca²⁺ back into SR.
Tropomyosin re-blocks actin sites → muscle returns to resting length (passive process)
Energetics of Contraction of Skeletal Muscle
ATP Roles:
Powers myosin detachment and resetting.
Fuels Ca²⁺ pumps in SR.
Creatine Phosphate: Rapidly regenerates ATP during short bursts.
Fatigue: Caused by ATP depletion, lactic acid buildup, or ion imbalances (K⁺, Ca²⁺).
Oxygen Debt: Excess post-exercise O₂ consumption to replenish ATP, clear lactate, and restore ions.
Types of Skeletal Muscle Contractions
Isotonic: shortens against a constant load (e.g., lifting weights).
Isometric: Muscle tension without shortening (e.g., holding a plank).
Eccentric: lengthens under tension (e.g. lowering a lift slowly)
Summation: rapid stimuli → increased force → graded muscle response
Tetanus: fused stimuli → sustained contraction/no relaxation → maximal muscle force
Twitch: Single contraction-relaxation cycle
Muscle Fiber Types
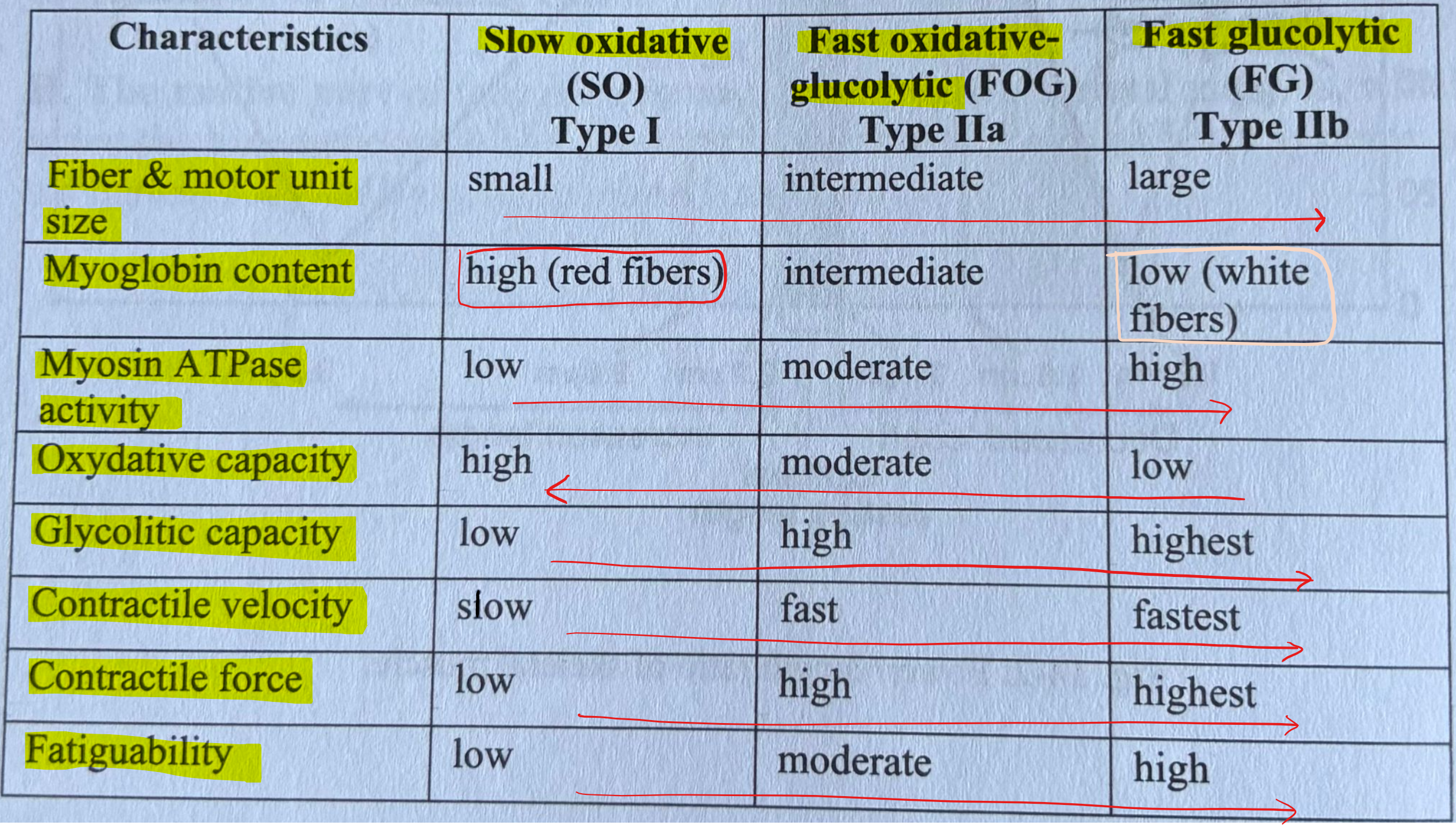
Skeletal Muscle Work & Fatigue
Work = Force × Distance.
Power = Work/Time.
Fatigue Mechanisms:
Peripheral: ATP depletion, lactic acid buildup, ionic imbalances (K⁺, Ca²⁺), glycogen loss.
Central: CNS inhibition (protective mechanism).
Recovery: Requires O₂ to restore ATP, clear lactate, and normalize ions.
Electromyography (EMG)
Principle: Records electrical activity of muscle fibers during contraction.
Motor Unit Action Potentials (MUAPs): Summed depolarization of fibers in a motor unit.
Clinical Uses:
Diagnose neuromuscular disorders (e.g., myasthenia gravis, ALS).
Assess muscle recruitment patterns in sports science.
Hypertrophy: Resistance training → increased myofibrils (not cell number).
Atrophy: Disuse → proteolysis (e.g., bed rest, denervation).
Denervation: Muscle fibers atrophy; replaced by fibrous tissue (contractures if untreated).
Rigor Mortis: Post-mortem ATP depletion → permanent cross-bridge attachment (stiffness).
Muscular Dystrophy: Genetic defects in dystrophin → sarcolemma instability.
Myasthenia Gravis: Autoimmune attack on ACh receptors → muscle weakness.
Functional Morphology of Smooth Muscles
No Sarcomeres: Actin/myosin arranged in lattice (attached to dense bodies).
Caveolae: Membrane invaginations (store Ca²⁺, similar to T-tubules).
Gap Junctions: Allow electrical coupling in unitary smooth muscle (e.g., gut).
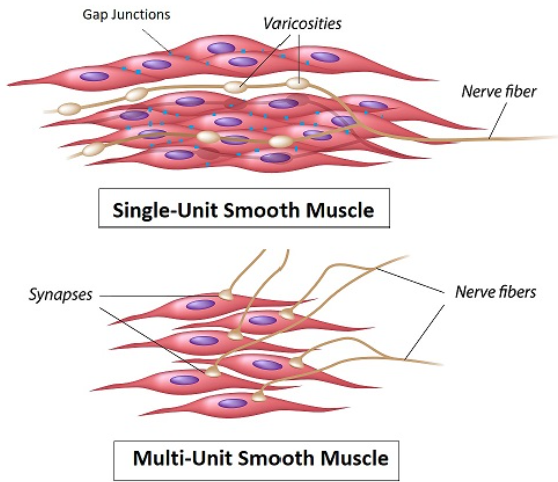
Unitary (Single-Unit) Smooth Muscle:
Structure: Sheets of fibers connected by gap junctions (electrical syncytium)
Control: Self-excitatory (pacemaker cells), hormones, stretch, or neurotransmitters
Example: Gut, uterus, blood vessels (visceral organs)
Multi-Unit Smooth Muscle:
Structure: Discrete, independent fibers (no gap junctions)
Control: Primarily by autonomic nerves (e.g., iris muscles, piloerector muscles)
Example: Adjusts pupil size or causes hair erection
Excitation & Electrophysiology of Smooth Muscle
Depolarization Mechanisms:
Autonomic Nerves: Release ACh/norepinephrine → activate receptors.
Pacemaker Cells: Generate slow waves (e.g., gut interstitial cells of Cajal).
Stretch: Opens mechanosensitive channels → depolarization.
Action Potentials:
Spike Potentials: Brief (10–50 ms) → phasic contractions (e.g., peristalsis).
Plateau Potentials: Sustained depolarization → tonic contractions (e.g., blood vessels).
Smooth Muscle - Mechanism of Contraction
Membrane depolarisation → open L-type voltage-gated calcium channels → extracellular Ca²⁺ enters the cell down its concentration gradient
Calmodulin-Dependent:
Intracellular Ca²⁺ binds calmodulin → activates myosin light-chain kinase (MLCK) → phosphorylates myosin → cross-bridge cycling.
Latch State: Myosin phosphatase dephosphorylates myosin → slow detachment → sustained tension.
Calcium Sources:
Extracellular: Via voltage-gated (L-type) or receptor-operated channels
Sarcoplasmic Reticulum: IP₃ or Ca²⁺-induced Ca²⁺ release
Latch Mechanism:
Sustained contraction with minimal ATP use.
Myosin remains attached to actin even after dephosphorylation (via myosin phosphatase).
Cross-bridge cycling is slower than in skeletal muscle → energy-efficient, prolonged contractions
Smooth Muscle Disorders
Hypertension: Overactivation of vascular smooth muscle (e.g., angiotensin II, endothelin).
Asthma: Bronchial smooth muscle contraction (treated with β₂-agonists like albuterol).
Erectile Dysfunction: Smooth muscle relaxation in penile arteries mediated by NO (treated with PDE5 inhibitors like sildenafil).
Tocolytics: Drugs that relax uterine smooth muscle (e.g., magnesium sulfate to delay preterm labor).