Chapter 26- carbonyls and carboxylic acids
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
56 Terms
what is the functional group of a carbonyl
c=o
what is an aldehyde functional group
-CHO
what is the functional group of a ketone
CO
oxidation of aldehydes
can be oxidised into carboxylic acids by being refluxed with Cr2O72-/ H+
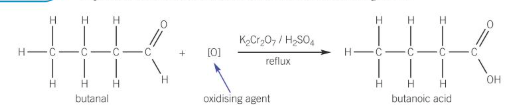
oxidation of ketones
they cannot undergo oxidation
why do carbonyls and alkenes react differently
although they both have a double bond, however
the c=c bond in non-polar
the c=o bond in polar
how does the polar nature of a c=o bond affect its reactivity
there is high electron density by the o
this gives the molecule the ability to react with nucleophiles. This nucleophile is attracted to the delta positive carbon resulting in the addition
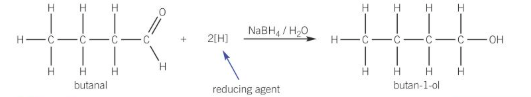
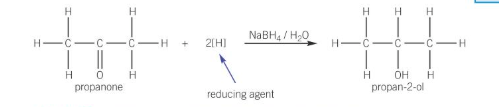
what compound is used to reduce carbonyl compounds
NaBH4 (warmed)
reduction of an aldehyde with NABH4
they are reduced to primary alcohols
reduction of ketones with NABH4
reduced to a secondary alcohol
what is HCN
hydrogen cyanide
a colourless, extremely poisonous liquid that boils slightly above room temperature
why isnt HCN used in the labs
it boils just above room temperature so cannot be used safely
what is used as a replacement to HCN in a lab
sodium cyanide and sulfuric acid
why is a reaction with HCN useful
it increases the length of the carbon chain
what is the mechanism for NaBH4
nucleophilic addition
steps of nucleophilic addition
1- the lone pair from the nucleophile is attracted to the delta positive carbon
2- a dative covalent bond is formed between the nucleophile and the carbon in the double bond
3- the pi bond of the c=o breaks by heterolytic fission forming a nagtively charge O intermediate
4- an oxygen donates an electron pair to a hydrogen in a near water molecule
5- this creates an alcohol on the main chain
what is the nucleophile for the reaction of NaBH4
hydride
what is the nucleophile for the reaction of NaCN/H+
cyanide ion
how can you detect a carbonyl compound
using 2,4-DNP (brady’s reagent)
positive 2,4-DNP result
yellow or orange precipitate
how can you distinguish between an aldehyde and a ketone
Tollen’s Reagent
positive result for Tollen’s reagent
a silver mirror
why does a silver mirror form from tollen’s solution
the silver ions act as an oxidising agent in the presence of ammonia
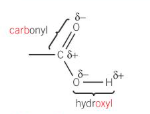
what is a carboxylic acid
COOH
common uses for carboxylic acids
medicines, fruit juices and vinegar
are carboxylic acids soluble
both the c=o and the o-h bond are polar so they can form hydrogen bonds with water
relationship between carboxylic solubility and chain length
as the number of carbon atoms increases, the solubility of the molecule decreases because the non-polar carbon chain has a greater effect on the overall polarity of the molecule
what type of acids are carboxylic acids
they’re weak acids which only partially dissociate in water to release H+ ions
what 2 types of reaction can carboxylic acids take part in
neutralisation with metal oxides, bases and carbonates
redox with metals
what do carboxylic acids become in reactions
carboxylate salts, with carboxylate ions
metals + carboxylic acid
redox
carboxylate salt and hydrogen
observation of a carboxylic acid + metal
effervesence and this disappearance of the metal
carboxylic acid + metal oxides
neutralisation
salt and water
carboxylic acid + alkalis
neutralisation
salt + water
carboxylic acid + carbonates
neutralisation
if the carboxylic acid is in excess a solid carbonate would disappear
how to differentiate carboxylic acids from other acids
the neutralisation reaction of carboxylic acid with carbonates
they are the only common acid, sufficiently acidic enough to react with carbonates
what is a derivative
a compound that can be hydrolysed to form the parent of a carboxylic acid
how to name an ester
remove -oic from parent carboxylic acid chain, replace with -oate
add the alkyl chain to the front
how to name an acyl chloride
remove oic from parent carboxylic acid chain, replace with -oyl chloride
what is an acid anyhydride
forms by the removal of a water molecule between two carboxylic acids
esterification
the reaction of n alcohol with a carboxylic acid to form an ester
conditions for ester formation
carboxylic acid warmed with conc H2SO4 which acts as a catalyst
acid hydrolysis of an ester
the ester is heated under reflux with dilute aqueous acid
the ester is broken down by water (with the acid acting as a catalyst)
products of an acid hydrolysis of an ester
carboxylic acid and an alcohol
alkaline hydrolysis of an ester
irreversible heating of an ester under reflux with aqueous hydroxide ions
product of alkaline hydrolysis of an ester
ester ion and an alcohol
acyl chloride preparation from a carboxylic acid
SOCl2
other products are SO2 + HCl
why are acyl chlorides useful
they are highly reactiveand can be easily converted into carboxylic acid derivatives with good yields
acyl chloride + alcohol
ester forms + HCl
acyl chloride + phenol
carboxylic acids arent reactive enough to react with phenols
acyl chlorides react to form phenyl esteres and HCl
acyl chloride + water
carboxylic acid + HCl gas
what type of reaction is the formation of a carboxylic acid from acyl chloride + H2O
a violent one
takes place with the evolution of dense steamy HCl fumes
acyl chloride + ammonia
ammonia acts as a nucleophile
forms a primary amide + ammonium chloride
primary amide + acyl chloride
forms a secondary amide
two ways to increase chain with mechanisms
nucleophilic addition with CN-
electrophilic substituion with alkylation
hwo to idnetify a cabronyl with bradys
bradys
orange ppt
melting poiunt
comapre to known data