9.3 Measuring Rate of Reaction
1/7
Earn XP
Description and Tags
Mg(s) + 2HCl(aq) ——> MgCl₂(aq) + H₂(g)
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
How will you measure rate of reaction in this reaction (it produces hydrogen)?
Gas syringe
What should the setup of the apparatus look like?
The reaction mixture should be in a conical flask with a stopper in it, attached to a gas syringe.
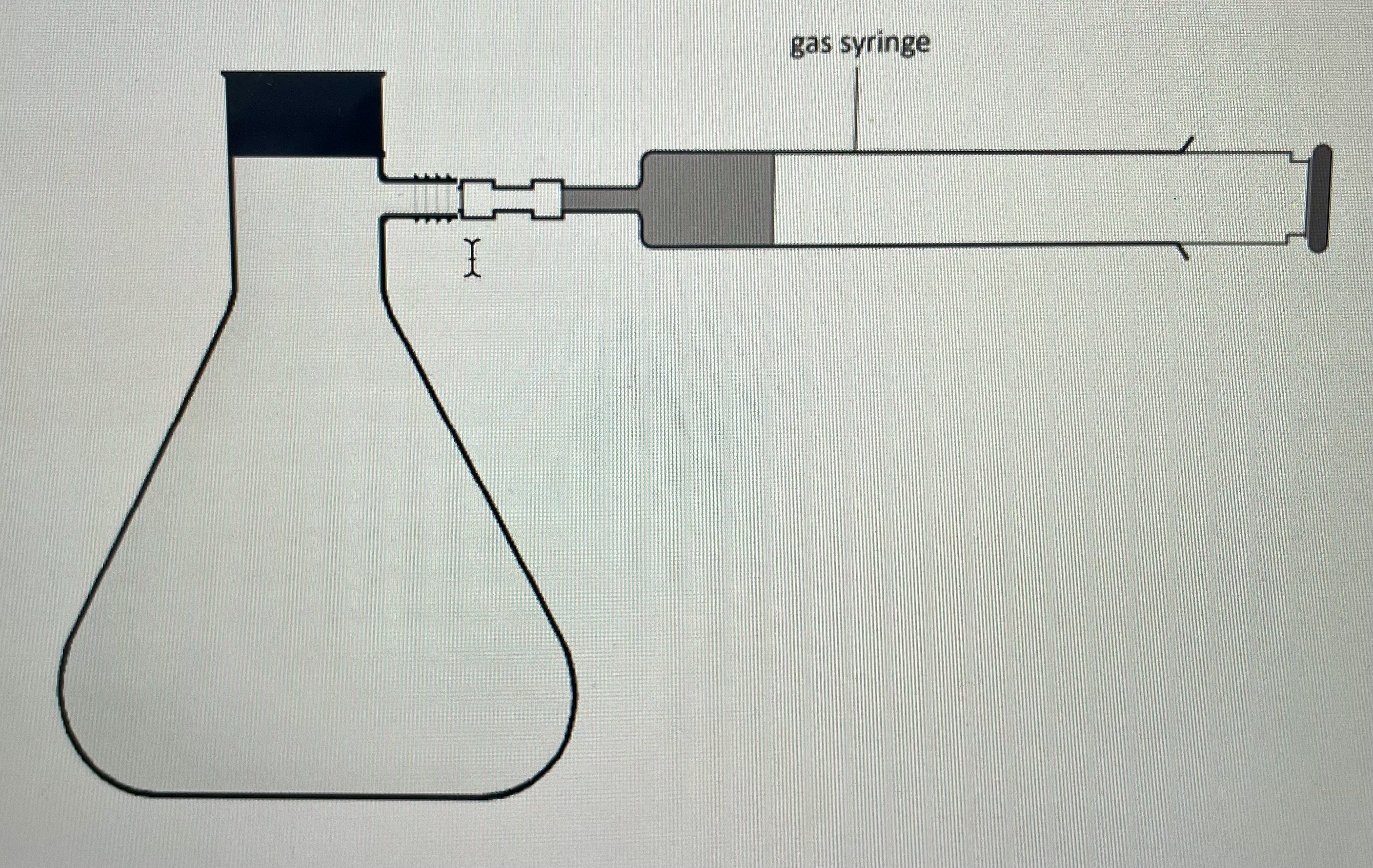
What should you do before putting magnesium into the conical flask?
Find its mass
(So that it can be used in the reaction equation - allows you to find moles)
Why should you put the stopper on the conical flask immediately after adding the magnesium?
To prevent the loss of hydrogen gas, to get a more accurate result
Why should you start the stopwatch immediately after adding the magnesium?
To ensure that you are recording the reaction time correctly
How often and for how long should you record the volume of gas produced?
Every 10 seconds
To a maximum of 3 minutes
What should you change when repeating the experiment?
Concentration of the HCl