5. Cell-cell interactions: TGF-beta and FGF signalling
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Common features in signal transduction pathways
What are the 3 common features in signal transduction pathways?
What is the mechanism of these features in order?
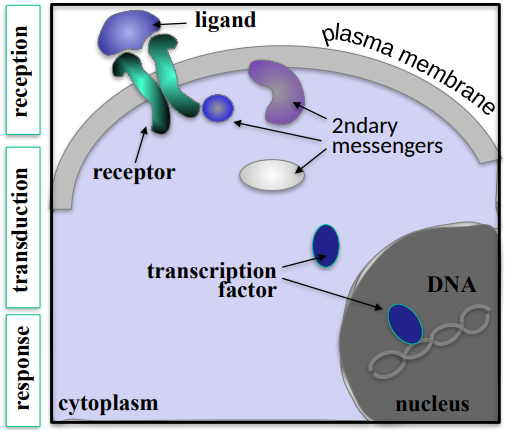
Reception, Transduction and Response.
Reception is where the ligand binds to a cell surface receptor and activates it, Transduction is when the receptor activation activates a cascade of secondary messenger molecules, whcih transmit the message form the membrane to the nucleus. The response is a transcription factor becoming activated, inducing the transcription of specific target genes.
The TGF-β (beta) superfamily
What is the TGF-β (beta) superfamily and what are some examples of their essential roles in early and late development?
How many dimeric ligands does the TGF-β superfamily consist of?
What is dimeric ligand?
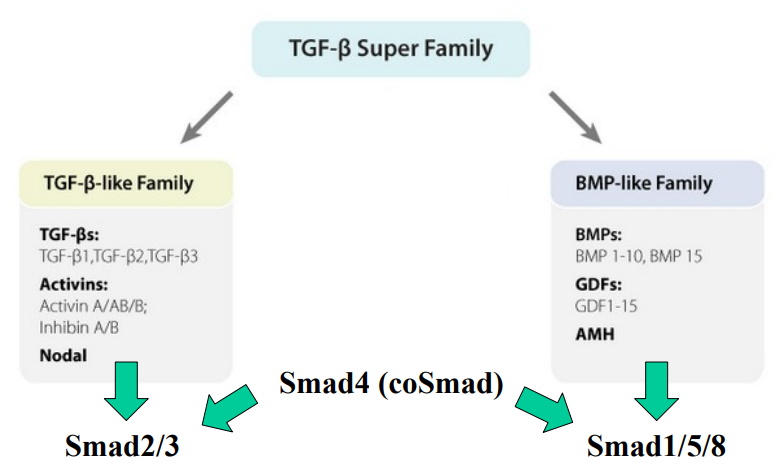
What are the 2 branches of the TGF-β superfamily for signalling?
What is the canonical (Smad-mediated) TGF-β signalling pathway?
What do the TGF-β-like ligands/members activate?
What does this activation lead to?
What do the BMP-like ligands/members activate?
What does this activation lead to?
What is Smad4?
What is Smad4 essential for?
This family encompasses a wide range of growth factors and cytokines. It has 2 sub-families. The TGF-β superfamily of ligands and their signalling pathway plays essential roles in several aspects of early & late development (e.g gastrulation, branching, postnatal growth and cancer).
The TGF-β superfamily consists of over 30 dimeric ligands.
Dimeric ligands: molecules composed of two identical monomers that bind to a target, such as a protein or enzyme, at two different sites.
The TGF-β-like Family and the BMP-like Family.
A signalling pathway which involves a series of steps where TGF-β binds to type II receptors, activating type I receptors, which then phosphorylate receptor-Smads. Phosphorylated receptor-Smads then associate with a common Smad and the complex translocates to the nucleus, where it regulates target gene expression.
TGF-β-like ligands/members primarily activate Smad2/3.
Smad2/3 then interacts with Smad4 (coSmad) to form a complex, which translocates to the nucleus to regulate gene expression.
BMP-like ligands/members activate Smad1/5/8.
Smad1/5/8 then interacts with Smad4 (coSmad) to form a complex, which translocates to the nucleus to regulate gene expression.
Smad4 acts as a "common mediator Smad" (coSmad).
Smad4 is essential for both the TGF-β-like and BMP-like signalling branches, as it partners with the receptor-activated Smads to form functional transcriptional complexes.
Overview of Smad signalling
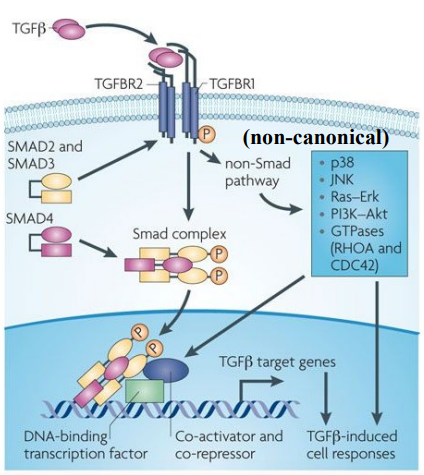
Reception:
What is the functional receptor complex for TGF-β composed of?
What type of molecules are the Type I & II TGF-β receptors
What is the mechanism of activation?
Transduction:
What do Type I receptors activate?
How is the Smad complex then formed?
What is a common theme in signal transduction involving the components, which leads to activation of the pathway?
Response:
What does the Smad complex function as in the nucleus?
What does the Smad complex then do resultantly?
What does this then recruit? (2)
What role do tissue-specific transcription factors play in modulation?
What is the result of TGF-β responses? (with 5 examples)
What about non-canonical pathways?
Reception:
The functional receptor complex for TGF-β is composed of a dimeric ligand (e.g TGF-β), Two Type I receptors (TGFBR1) and Two Type II receptors (TGFBR2).
Both TGFBR1 and TGFBR2 are serine/threonine kinases, meaning they phosphorylate target proteins on serine or threonine residues.
TGF-β ligand first binds to the Type II receptor, this then activates the Type I receptor by the phosphorylation of multiple serines/threonines in the GS domain (a specific region within the Type I receptor).
Transduction:
Type I receptors activate (phosphorylate) specific Smad proteins (e.g Smad2/3), which act as secondary messengers, relaying the signal from the cell surface into the cell's interior.
Phosphorylates Smad2/3 then bind to Smad4, forming a stable Smad complex.
A common theme in signal transduction is that components of the pathway change their subcellular localisation, specifically the Smad complex, which moves from the cytoplasm to the nucleus. This change in location is essential for activating the downstream pathway.
Response:
The Smad complex translocates into the nucleus. Once in the nucleus, the Smad complex functions as a DNA-binding transcription factor.
The Smad complex will bind to specific DNA recognition sequences within the promoter regions of target genes.
Then recruits other nuclear proteins: co-activators to enhance gene expression and co-repressors to suppress gene expression.
Tissue-Specific Modulation: tissue-specific TFs play a critical role in modulating Smad binding to target genes. This means the exact set of genes activated or repressed by TGF-β can vary significantly depending on the cell type and its specific transcriptional machinery. Allowing for diverse & context-dependent cellular responses to the same TGF-β signal.
The outcome is a range of TGF-β-induced cellular responses (e.g processes like cell growth inhibition, differentiation, apoptosis, epithelial-mesenchymal transition (EMT), and regulation of the immune system).
While Smad signalling is the primary canonical pathway, TGF-β receptors can also activate parallel “non-Smad pathways”, such as the Ras-Erk pathway and a range of GTPases.
Control of BMP signalling
How is control of BMP signalling operated?
What are the components of the BMP signalling pathway?
What is the mechanism of BMP signalling for the Smad complex to end up in the nucleus?
What does the Smad complex do in the nucleus?
What is the main way of controlling BMP signalling through inhibition?
Explain this.
What are some examples of this molecule that can control BMP signalling thorugh inhibition? (3)
What is another way BMP signalling may be controlled through inhibition?
Control of BMP signalling is operated through inhibition. Unlike some signalling pathways that are primarily regulated by activation, BMP signalling is extensively controlled at multiple levels, particularly through various inhibitory mechanisms.
Ligands (BMPs and GDFs), Type II receptors and Type I receptors.
BMP/GDF ligands bind to Type II receptors, which then activates Type I receptors (phosphorylation). Activated Type I receptors then phosphorylate Smad1/5/8 which partner with Smad4. This Smad complex translocates to the nucleus.
In the nucleus, the Smad1/5/8/4 complex acts as a transcription factor, often associating with other TFs and co-activators/co-repressors, to regulate the transcription of target genes (mRNA).
Ligand antagonists (ligand traps).
Ligand antagonists (ligand traps): Secreted proteins that bind directly to BMP ligands in the extracellular space, they preveent the ligands from activating their receptors, “trapping” the ligand.
Chordin, Noggin and Follistatin
Inhibitory Smads (e.g Smad6/7) are often induced by active BMP signalling, creating a negative feedback loop, intefering with the formation of the receptor-Smad complex, they may also compete with receptor-Smads for binding to Smad4.
Pathway Activation Visualisation
How could pathway activation be visualised?
What experimental technique would most likely be used?
How would it visualise pathway activation?
What is used to visualise the expression of specific target genes?
What is an example of a model organism that wa
Pathway activation can be visualised by phosphorylated Smad detection.
Immunofluorescence could be used to visualize and track the activation of signalling pathways. Immunofluorescence uses antibodies conjugated with fluorescent dyes to detect specific proteins or antigens within cells or tissues.
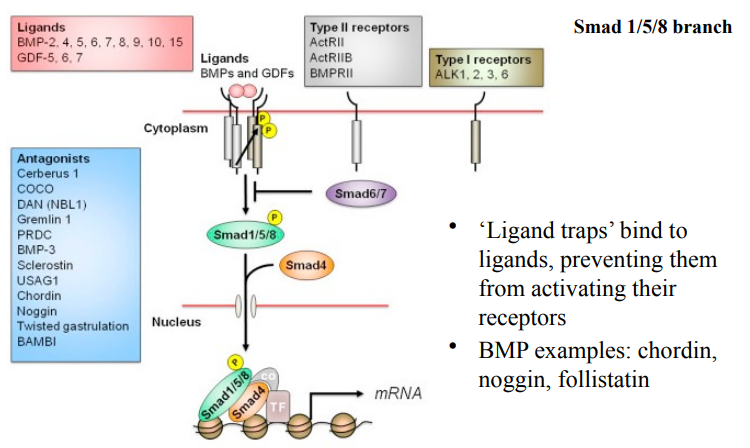
By using antibodies specific for the phosphorylated form of these proteins (e.g., pSmad1/5), researchers can directly visualize where and when the pathway is active. The presence of pSmad1/5 indicates active BMP signalling, since Smad 1/5 are the receptor-Smads primarily activated by the BMP Smad pathway.
Immunoflueorscence is also used to visulaise expression of genes that are often targetr of BMP signalling - e.g Chordin and NOggin which are known BMP antagonists. Their expression often marks regions where BMP signalling is being inhibited or patterned.
A zebrafish tailbud (in image) was visualised at 2 different developmental stages (3 somites vs 12 somites). The visualisation showed the dynamic regulation & patterning of BMP signalling, by its antagonists (chordin, noggin) in the developing zebrafish tailbud.
Tools for analysis of TGF-β signalling
How can we use genetic engineering?
What are 2 examples of functional modifications we can engineer?
Explain these.
What does the W/T Type I receptor stand for?
What effect would kinase dead receptors have on signalling?
What effect would constitutively active receptors have on signalling?
So how does this example of genetic engineering aid in the understanding of the roles of TGF-β signalling?
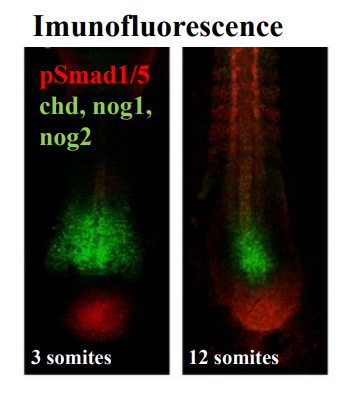
Using genetic engineering we can generate DNA that encodes a mutated receptor. By creating modified receptors that either lose function or gain constitutive activity, it allows researchers to probe the pathway's roles.
Kinase dead and Constitutively active.
Kinase dead: mutated version of the Type I receptor where the kinase domain is rendered non-functional - the receptor cannot phosphorylate downstream Smads.
Constitutively active: mutated version where the receptor is designed to be always active, even in the absence of ligand or Type II receptor phosphorylation.
WT Type I receptor: serves as a control for comparison with mutated versions.
Kinase dead receptors would act as a dominant-negative inhibitor. When expressed, it can still bind the Type II receptor and potentially sequester it, but it cannot transmit the signal. This effectively blocks or reduces normal TGF-β signalling, mimicking a loss of function in the pathway.
Constitutively active receptors kinase domain is continuously active. When expressed, it leads to continuous activation of the downstream Smad pathway, regardless of normal regulatory cues. This mimics a gain of function in the pathway.
The engineered DNA is then expressed in cells (e.g., in cell culture) or directly into an embryo (e.g., via injection into a developing organism). The mutated receptors interfere with the normal TGF-β signalling pathway through either loss of fucntion or gain of function. By observing the resulting cellular/developmental phenotypes (e.g., changes in cell proliferation, differentiation, migration, tissue patterning) researchers can deduce the specific role of TGF-β signalling in those processes.
Using model organisms to dissect clinically relevant signalling pathways
Which signaling pathway is required for mesoderm formation and what molecules mediate this in vivo?
What are squint (Sqt) and cyclops (Cyc)?
What is oep-cripto?
What do oep-cripto mutations lead to?
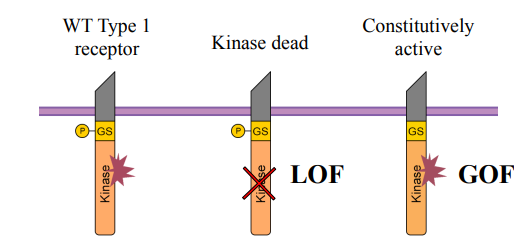
Describe the general phenotype seen in the cyclops;squint mutant zebrafish embryos.
Describe the general phenotype seen in the MZoep (maternal zygotic one-eyed pinhead) mutant zebrafish embryos.
What conclusion can be drawn by comparing the phenotypes of the cyc;sqt and MZoep mutants?
Smad2/3 signalling is required ofr mesoderm formation, it is mediated by Nodal ligands in vivo (Zebrafish, Xenopus).
Squint (Sqt) and Cyclops (Cyc) are zebrafish Nodal ligands.
Oep-Cripto (products of the oep gene) are transmembrane proteins which are co-receptors for Nodal ligands (oep = One-eyed pinhead gene).
Mutations in oep-cripto can lead to developmental defects.
Cyclops;squint mutant zebrafish embryos: Severe developmental defects, particularly in A/P patterning, likely leading to severe head and trunk abnormalities (e.g., cyclopia, "pinhead" appearance).
MZoep mutant zebrafish embryos: Severe developmental defects, particularly in A/P patterning, similar to the cyclops;squint mutant, characterized by severe head and trunk abnormalities.
Nodal ligands (Squint/Cyclops) and their essential co-receptor (oep-cripto) are both critical for proper early embryonic development, as mutations in either lead to similar severe phenotypes.
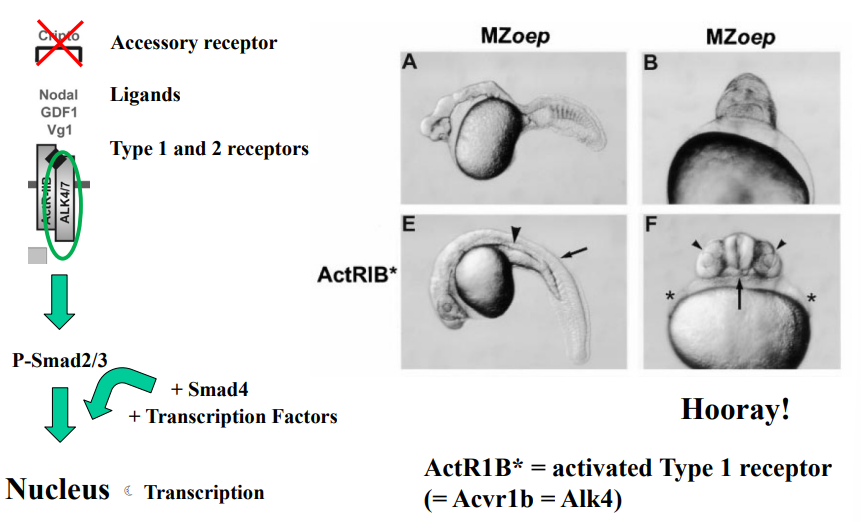
oep mutants are rescued by injection of activated Type 1 receptor mRNA
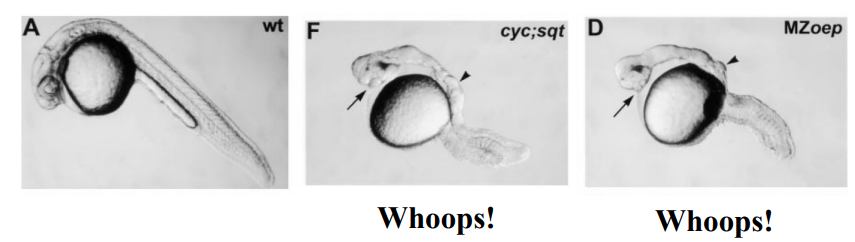
What is activated Type I receptor mRNA?
What does it mean for oep mutants to be rescued by the injection of this mRNA?
What does this result suggest about oep mutants? (2)
messenger RNA (mRNA) that, when translated in the cell, will produce a Type I receptor protein that is already in an active state, even without the normal upstream signals (like Nodal ligand binding or Type II receptor phosphorylation).
When the mRNA encoding the activated Type I receptor is introduced into the oep mutant embryos (typically by microinjection), the developmental defects caused by the oep mutation are alleviated or corrected. The embryos develop more normally, or at least show a significant improvement in their phenotype.
The Type I receptor is a downstream component of the Nodal pathway. This suggests the oep mutant impacts upstream of Type I receptor activation and also that the downstream components of the TGF-β pathway (like Smads) are still functional in oep mutants.
What does the existence of several inhibitors allow for in TGF-β signalling?
The existence of several inhibitors (such as ligand antagonists and Inihibitory Smads) allows for fine tuning of the signal output, an important feature of cell fate specification in early embryogenesis.
The diverse array of inhibitors in TGF-β signaling, acting at different levels of the pathway (extracellularly, intracellularly), allows for:
Spatial patterning: Precisely defining where signals are active.
Temporal control: Regulating the duration and intensity of signals.
Contextual responses: Allowing cells to interpret signals differently based on their environment.
This multifaceted control is absolutely essential for the highly orchestrated and precise processes of CELL FATE SPECIFICATION during early embryogenesis.
Receptor Tyrosine Kinases (RTKs)
What are receptor tyrosine kinases?
How many RTK genes have been identified in the human genome, and how many subfamilies have these been grouped into?
What is the specificity of a receptor for a ligand in RTKs?
What do RTKs exist as when unbound, and which specific receptor is the only exception?
What are some examples of RTKs?
A large family of cell surface signalling receptors.
58 RTK genes identified in human genome, grouped into 20 subfamilies.
Some receptors are specific for one ligand and vice-versa. Other receptors interact with many ligands and vice-versa.
Mostly exist as monomers when unbound (except Insulin receptor).
EGF receptors, Insulin receptors, PDGF, FGF receptors, e.t.c.
Receptor Tyrosine Kinases (RTKs)
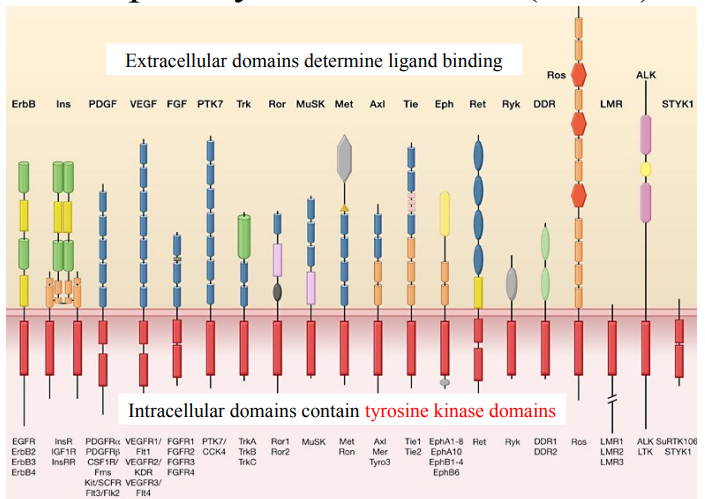
What is the structure of RTKs?
What are extracellular domains and what do they determine?
What does diversity of extracellular domains mean?
What are intracellular domains and what do they contain?
What happens between the extracellular domain and the intracellular domain when a ligand binds to the extracellular domain?
What roles do they have in cell biology? (6)
RTKs are transmembrane proteins, they are embedded in the cell’s plasma membrane, in the lipid bilayer.
Extracellular domains are the portions of the receptor that extend outside the cell. They determine ligand binding. Ligand binding then initiates a signalling cascade.
There is a wide variety of shapes and arrangements of extracellular domains across different RTK subfamilies. This structural diversity accounts for the specificity of ligand binding for each RTK.
Intracellular domains are the portions of the receptor that extend into the cell’s cytoplasm. They contain tyrosine kinase domains which possess enzymatic activity - the ability to phosphorylate tyrosine residues on other proteins (including the RTK itself).
When a ligand binds to the extracellular domain, it typically causes the RTK to dimerise and then undergo autophosphorylation (phosphorylation of its own tyrosine residues) on these intracellular kinase domains. This autophosphorylation creates docking sites for other signalling proteins, initiating downstream signalling pathways.
Roles in cell biology:
Cell growth and proliferation
Cell differentiation
Cell survival
Metabolism
Cell migration
Tissue repair and development

Canonical RTK receptor activation
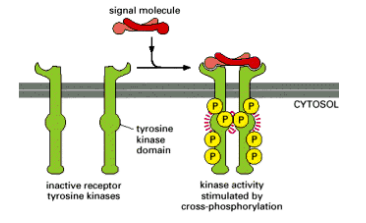
What is the process that allows kinase domains on the RTK to cross-phosphorylate?
What does cross-phosphorylation do? (3)
The ligand usually dimerises, facilitating receptor dimerisation (or oligomerisation). Once positioned correctly, the kinase domains cross-phosphorylate each other.
Cross-phosphorylation often:
Increases the activity of the kinase
Stabilises the receptor in the active state (ligand independent)
Causes the kinase to phosphorylate other tyrosines in the receptor to create “docking sites”
Canonical RTK transduction
What is canonical RTK transduction?
What is the mechanism for RTK transduction after ligand binding, receptor dimerisation and cross-phosphorylation has occurred?
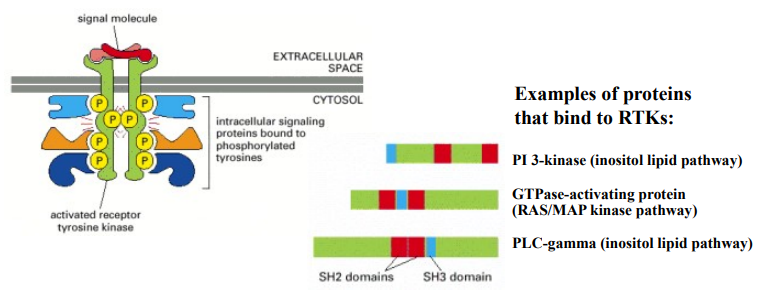
What are SH2 domains, and explain how they recognise/bind to phosphorylated tyrosine residues?
What are some examples of proteins that bind to RTKs?
When RTKs transmit signals from the outside to the inside of a cell, using tyrosine phosphorylation and the binding of downstream signalling proteins.
The specific tyrosine residues that have been phosphorylated via cross-phosphorylation now act as docking sites for downstream signalling proteins because the phosphate groups create specific binding motifs that recruit other proteins form the cytoplasm. Once bound, they are often activated themselves (via phosphorylation or conformational change) and relay the signal further into the cell, initiating a cascade of events.
SH2 domains are a common protein domain found in many signalling proteins, they are specifically designed to recognise and bind to phosphorylated tyrosine residues within a particular amino acid sequence context. The SH2 recognises the short phosphopeptide: phosphotyrosine - glutamic acid - glutamic acid - isoleucine. Different SH2 domains have slightly different specificities, allowing them to bind to distinct phosphorylated tyrosine sites on different RTKs or even on the same RTK at different positions.
PI 3-kinase (inositol lipid pathway), GTPase-activating protein (RAS/MAP kinase pathway) & PLC-gamma (inositol lipid pathway).
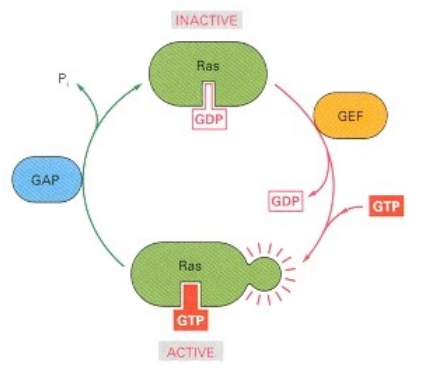
Ras pathway - An example of how docking leads to signal transduction
What are the two states of Ras and what nucleotide is bound in each?
What proteins switch Ras ON and how do they do it?
What proteins switch Ras OFF and how do they do it?
How does an activated Receptor Tyrosine Kinase (RTK) initially connect to inactive Ras?
What is Sos's role and how does it activate Ras?
What happens to Ras after it's activated by Sos?
Inactive state: Ras-GDP; Active state: Ras-GTP.
GEFs (Guanine Nucleotide Exchange Factors) switch Ras ON; They promote the release of GDP, allowing GTP to bind (GTP is more abundant).
GAPs (GTPase-Activating Proteins) switch Ras oFF; They stimulate Ras's intrinsic GTPase activity, hydrolyzing GTP back to GDP.
An adapter protein (GRB2), containing an SH2 domain, binds to phosphorylated tyrosines on the activated RTK.
Sos is a GEF. It binds to GRB2 (via SH3 domains) and is brought to the membrane. Sos then promotes the dissociation of GDP from Ras.
GTP binds to Ras, activating it (Ras-GTP), and it then dissociates from Sos to signal to downstream effectors.
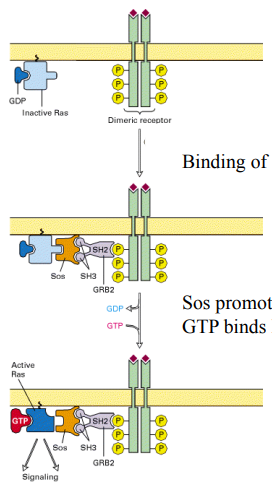
Signal transduction downstream of RTKs
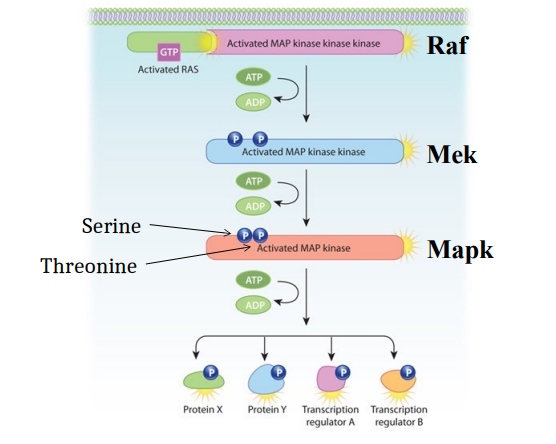
How can RTK signal transduction lead to the MAPK cascade?
Describe the initiation by activated Ras.
Describe what then happens to Raf.
Describe what then happens to Mek.
Describe what then happens to Mapk.
What are the cellular outcomes of this MAPK cascade?
RTK activates the MAPK cascade, a major downstream signalling pathway through GEFs linked to RTKs which activates Ras (located at the cell membrane).
Activated Ras (Ras bound to GTP), which is located at the cell membrane recruits Raf to the membrane, directly binding to and activating Raf, the first kinase in the cascade.
Activated Raf (a serine/threonine kinase) phosphorylates and activates the next kinase in the cascade, Mek. This phosphorylation event typically consumes ATP (converting it to ADP).
Activated Mek phosphorylates and activates the final kinase in the cascade, Mapk. This phosphorylation also consumes ATP.
Mapk (aka ERK1/2) is a serine/threonine kinase, for Mapk’s full activation Mek needs to phosphorylate Mapk on both serine and threonine residues (dual phosphorylation). It is the final kinase in this particular MAPK cascade. Once activated, Mapk can translocate into the nucleus and/or phosphorylate a variety of downstream target proteins, such as transcription factors, allowing DNA binding to specific DNA sequences to regulate the expression of target genes. The cascade can also lead to the regulation of other cytoplasmic proteins by phosphorylation.
Cellular outcomes such as: Cell proliferation (growth and division), Cell differentiation, Cell survival and Gene expression changes.
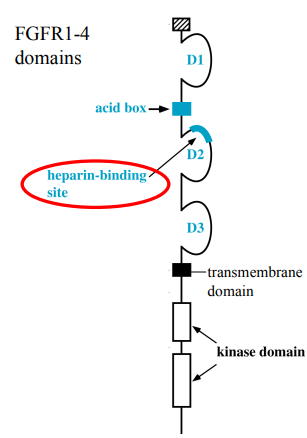
FGF (fibroblast growth factor) receptor family
How many members are there in the FGF ligand family?
How many FGF receptors are there?
In what 3 ways can FGFs act (modes of action)?
What type of receptor are FGFs?
Describe the extracellular domain of FGFs.
Which Ig-like domains do FGF ligands primarily bind to?
The family of FGF ligands contains 22 members.
All FGFs signal through just 4 receptors: FGFR1, FGFR2, FGFR3, FGFR4.
Paracrine (act locally on neighbouring cells), Intracrine (act within same cell), Endocrine (act as circulating hormones).
FGF receptors are Receptor Tyrosine Kinases (RTKs). This means they have an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular kinase domain.
Composed of 3 Immunoglobulin (Ig)-like domains: D1, D2, and D3. There's an "acid box" region between D1 and D2, which contributes to receptor function. An important feature is the heparin-binding site near the D2 domain (important when talking about HSPGs).
FGF ligands primarily bind to the D2 and D3 Ig-like domains.
FGFR activation by paracrine FGFs
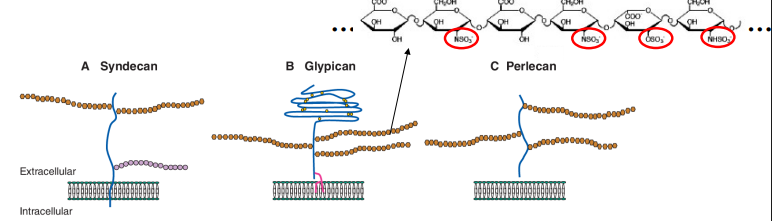
What are HSPGs?
What do HSPGs consist of? (2)
How can each heparin sugar be modified?
Explain the polyanionic nature of heparan sulfate chains.
What could result from modification of the heparan sugar chains?
So, what is the role of HSPGs in FGFR activation, and what 3 things do they do?
HSPGs are important extracellular modifiers of cell-cell signalling, particularly for growth factors like FGFs.
HSPGs consist of: a protein core (can be transmembrane, tethered or secreted) and long chains of heparan sulfate sugars (complex polysaccharide chains attached to the protein core).
Each sugar unit in the heparan sulfate chain can be modified in many different ways. A critical modification is sulphation. Different patterns of sulphation create unique binding sites.
Due to the numerous sulfate groups, heparan sulfate chains are highly polyanionic (negatively charged). This charge is important for their interactions with positively charged proteins.
The specific pattern of sugar modifications, particularly sulphation, can create a "code" that forms precise binding sites for specific proteins, such as different FGF ligands (e.g., FGF2). This allows for specificity in FGF-HSPG interactions.
HSPGs act as a co-receptor for FGFs. They help to:
Increase local concentration of FGF ligands near the cell surface.
Promote dimerization of FGF-bound FGFRs, which is essential for receptor activation and initiation of downstream signaling.
Stabilise the FGF-FGFR complex.
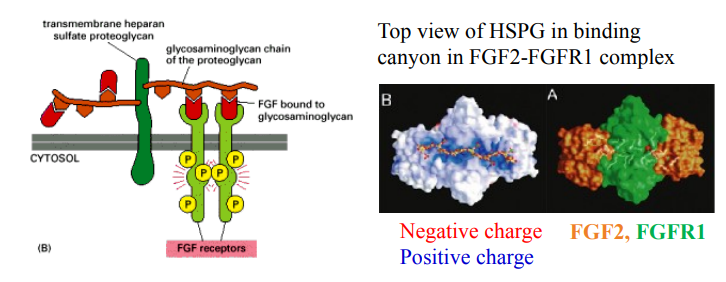
FGFR activation by paracrine FGFs
What key molecules are required for paracrine FGFs to activate FGFRs?
How do HSPGs help activate FGFRs (molecular mechanism)?
What happens between negatively charged HSPG and poisitively charged FGF complexes (e.g FGF2-FGFR1 complex)?
How do paracrine FGFs differ from endocrine FGFs in terms of HSPG affinity and action?
Heparan Sulfate Proteoglycans (HSPGs) are required as co-receptors.
FGF first binds to the HSPG chain; this helps dimerize two FGFRs, leading to their autophosphorylation/activation and initiation of signalling.
Negatively charged HSPG interacts with positively charged FGF2 and FGFR1, forming the signalling complex.
Paracrine FGFs have high affinity for HSPGs, are retained locally, and act locally. Whereas, Endocrine FGFs have low affinity for HSPGs, allowing them to diffuse into the bloodstream to act distantly.
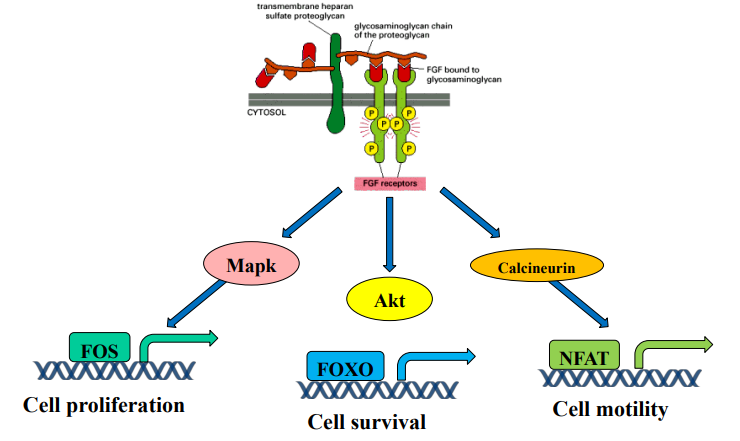
FGF Signaling - Pathway Diversification
What is the main concept illustrated by FGF signaling activating multiple pathways?
Which pathway leads to Cell Proliferation? What key transcription factor is involved?
Which pathway leads to Cell Survival? What key transcription factor is regulated?
Which pathway leads to Cell Motility? What key transcription factor is involved?
Signal diversification / Pathway branching: One receptor/ligand can trigger multiple distinct cellular responses.
MAPK pathway (via activated Mapk); Activates FOS.
Akt pathway; Inhibits FOXO.
Calcineurin pathway; Activates NFAT.
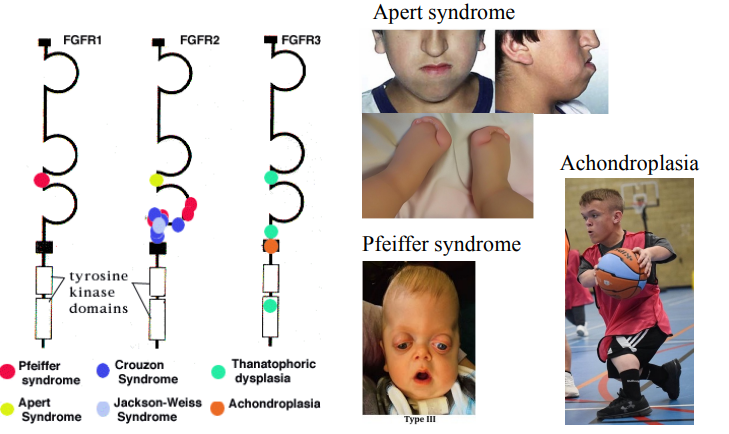
Mutations in FGFR are associated with many human diseases
List a syndrome associated with mutations in both FGFR1 and FGFR2. Describe the key features.
List a syndrome associated with mutations in FGFR3. Describe the key features.
Pfeiffer syndrome - Craniosynostosis (premature skull fusion) and distinct facial features (e.g., wide-set eyes).
Achondroplasia - Disproportionate short stature with short limbs, normal trunk, large head, and prominent forehead.