BBH 203 Exam 2
1/199
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
200 Terms
Synapses
The junction between neurons
Chemical Synapses
Presynaptic neuron secretes neurotransmitters into synaptic cleft, which bind to postsynaptic neuron
Electrical Synapses
Direct exchanges of ions/molecules at physical junctions between two neurons
Types of chemical synapses (Structural)
1. Axodendritic
2. Axosomatic
3. Axoaxonic
4. Dendrodendritic
Types of chemical synapses (functional)
1. Excitatory
2. Inhibitory
Neural Circuit
A group of neurons and their synaptic connections
Convergence (properties of neural circuits)
Many presynaptic neurons send signals to one or a few post-synaptic neurons
Divergence (properties of neural circuits)
One or few presynaptic neurons send signals to many postsynaptic neurons
Neurotransmitter
The chemical released from the presynaptic neuron that serves as the basis for the chemical synaptic communication
Criteria for a neurotransmitter at a given synapse
1. Substance must be present in presynaptic neuron
2. The substance must be released in response to presynaptic depolarization, and the release must be Ca2+ dependent
3. Specific receptors for the substance must be present on the postsynaptic cell
Amino Acid Neurotransmitters
Glutamate (Glu)
GABA
Glycine (Gly)
Monoamines
Serotonin
Dopamine
Norepinephrine
Synaptic transmission, synthesis, release, and binding
1. Most neurotransmitters are synthesized in presynaptic axon terminal and are stored in synaptic vesicles
2. An AP reaches the axon terminal to activate voltage-gated Ca2+ channels
3. Ca2+ moves down its electrochemical gradient to enter the axon terminal
4. Increased intracellular Ca2+ concentration promotes vesicle exocytosis via interactions between synaptotagmin and SNARE complexes
5. Neurotransmitters diffuse across synaptic cleft and bind to postsynaptic receptors
T/F: Post synaptic potentials are graded
True
Excitatory Postsynaptic Potential (EPSP)
Increase probability of postsynaptic action potentials
EPSPs depolarize the postsynaptic neuron (Vm gets more positive)
Inhibitory Postsynaptic Potential (IPSP)
Decrease probability of postsynaptic action potentials
IPSPs almost always hyperpolarize the postsynaptic neuron (Vm gets more negative)
Spatial summation
PSPs arriving in rapid succession from different presynaptic sources sum
Temporal summation
PSPs arriving in rapid succession from the same presynaptic source sum
What determines the postsynaptic effect(s) of the neurotransmitter?
The neurotransmitter receptor
Varieties of neurotransmitter receptors
1. Ionotropic receptors
2. Metabotropic receptors
Ionotropic Receptors
-Complexed with ion channels that allow one of more types of ions to pass
- Rapid and transient action, causing a PSP
Metabotropic Receptors
-Most are G protein-coupled receptors
- Two general varieties
(1) Indirect gating of ion channels (2) Activation of 2nd-messenger pathways
-Slow and sustained action, with many potential responses (Including PSPs)
What is the problem with letting synaptic release/binding continue unchecked?
Neurotransmitters must somehow be removed from the synaptic cleft
Two mechanisms for removing neurotransmitters from the synaptic cleft
1. Reuptake- Presynaptic neuron or astrocytes reabsorb NT (Ex: serotonin, dopamine, norepinephrine)
2. Degradation- NTs are broken down
(Ex: acetylcholine)
Glutamate and GABA can be removed by either process
Summary of Synaptic Transmission
1. Most NTs are synthesized in presynaptic axon terminal and are stored in synaptic vesicles
2. An AP reaches the axon terminal to activate voltage-gated Ca2+ channels
3. Ca2+ moves down its electrochemical gradient to enter the axon terminal
4. Increased intracellular Ca2+ concentration promotes vesicle exocytosis via interactions between synaptotagmin and SNARE complexes
5. NTs diffuse across synaptic cleft and bind to postsynaptic receptors
6. NT-receptor binding triggers some effect in the postsynaptic neuron
7. NTs unbind and are removed from the synaptic cleft through reuptake and/or degradation
Acetylcholine (ACh)
Two major receptors:
1. nictotinic (nAChRs, ionotropic) 2. Muscarinic (mAChRs, metabotropic)
CNS: synthesized in basal forebrain and pons
PNS: synthesized at neuromuscular junctions and the autonomic nervous system
Clinical correlates: dementia, myasthenia gravis, dysregulation of autonomic nervous system
Glutamate (Glu)
Most abundant excitatory NT in CNS
-synthesized in brain and spinal cord
Involved in many neural processes and connected to synaptic plasticity
Major receptors:
Ionotropic Receptors: AMPA, NMDA
Metabotropic Receptors: mGluRs
Clinical correlates: Excitotoxicity
GABA (gamma-aminobutyric acid)
The most abundant inhibitory NT
Major receptors:
1. GABAaRs (ionotropic)
2. GABAbRs (metabotropic)
Clinical correlates: Alterations in Huntington's & Parkinson's disease, Alzheimer's disease and other dementia's, and schizophrenia
Serotonin (5-HT)
Functionally prolific: descending pain control, mood, appetite, arousal, and aggression
At least 14 types of receptors (almost all metabotropic)
Synthesized in Raphe Nuclei of brainstem
Clinical correlates: Selective serotonin reuptake inhibitors (SSRIs) prescribed to treat depression, anxiety, and personality disorders
Dopamine (DA)
Functions: motor action selection, reward, general cognition
At least 5 different types of receptors (metabotropic)
Synthesized in midbrain
Clinical correlates: Parkinson's disease, dementia w/ Lewy bodies, schizophrenia, all psychoactive drugs
Norepinephrine (NE)
5 different receptor subtypes (metabotropic)
CNS: Synthesized in locus coeruleus (pons) and areas of the midbrain
- Involved in alertness, arousal, feeding, and reward
PNS: Synthesized in sympathetic division of autonomic nervous system
Drug
Any substance that alters an organism's physiology when ingested (other than nutrients considered necessary to normal functioning)
Pharmacology
The interaction between drugs and living organisms
Neuropharmacology
The interaction between psychoactive drugs and the nervous system
Psychopharmacology
The interaction between psychoactive drugs and cognitive processes
Ligand
A chemical binding to a receptor molecule
T/F: Neurotransmitters are the endogenous ligands of the nervous system
True
Exogenous ligands
Drugs binding to receptors
Psychoactive drug
A drug binding to one or more varieties of NT receptor that causes neurophysiological changes
Agonist
A ligand that binds to and activates a receptor, mimicking the action of the NT
Inverse Agonist
An agonist exerting the opposite effect of the NT
Antagonist
A ligand that binds to and blocks other ligands from binding to a receptor
Competitive ligand
A ligand that directly competes with a receptor's NT at the receptor's primary binding site
-Proper agonists and antagonists are competitive
Allosteric modulator
A noncompetitive ligand that binds to a non-primary (modulatory) receptor binding site
-AKA neuromodulator
-Doesn't compete with NT at primary binding site
-Can be positive (agonist-like) or negative (antagonist-like)
Pharmacodynamics
The factors that affect the relationship between a drug and its target receptors
Efficacy
The extent to which a drug activates a receptor after binding
Agonists= high
Antagonists= low or none
Binding Affinity
How strongly a drug binds to its receptors
High affinity drug = effective at lower concentrations
Low affinity drug = effective only at higher concentrations
Do neurotransmitters have a low or high binding affinity?
They are low-affinity ligands
Pharmacokinetics
The factors that affect the movement of a drug into, through, and out of the body
The blood-brain barrier (BBB) affects pharmacokinetics in the CNS, psychoactive drugs can permeate the BBB
Tolerance
Repeated exposure to the same dose of a drug resulting in a lesser effect
Metabolic tolerance
Repeated exposure to a drug enhancing the body's efficiency in clearing it
Functional tolerance
Repeated exposure to a drug leading to reduced efficacy via synaptic plasticity
Receptor downregulation if the drug is agonist
Receptor upregulation if the drug is antagonist
Synaptic plasticity
The nervous system is able to tune synapses
Cross-tolerance
Tolerance of one drug generalizing to other chemically related drugs
Sensitization
Repeated exposure to a drug leading to increased efficacy
Postsynaptic drugs
1. Receptor antagonists
2. Receptor agonists
-Neuromodulators (GABAa receptors)
3. Regulators of postsynaptic receptor density
4. Modulators of intracellular signaling cascades
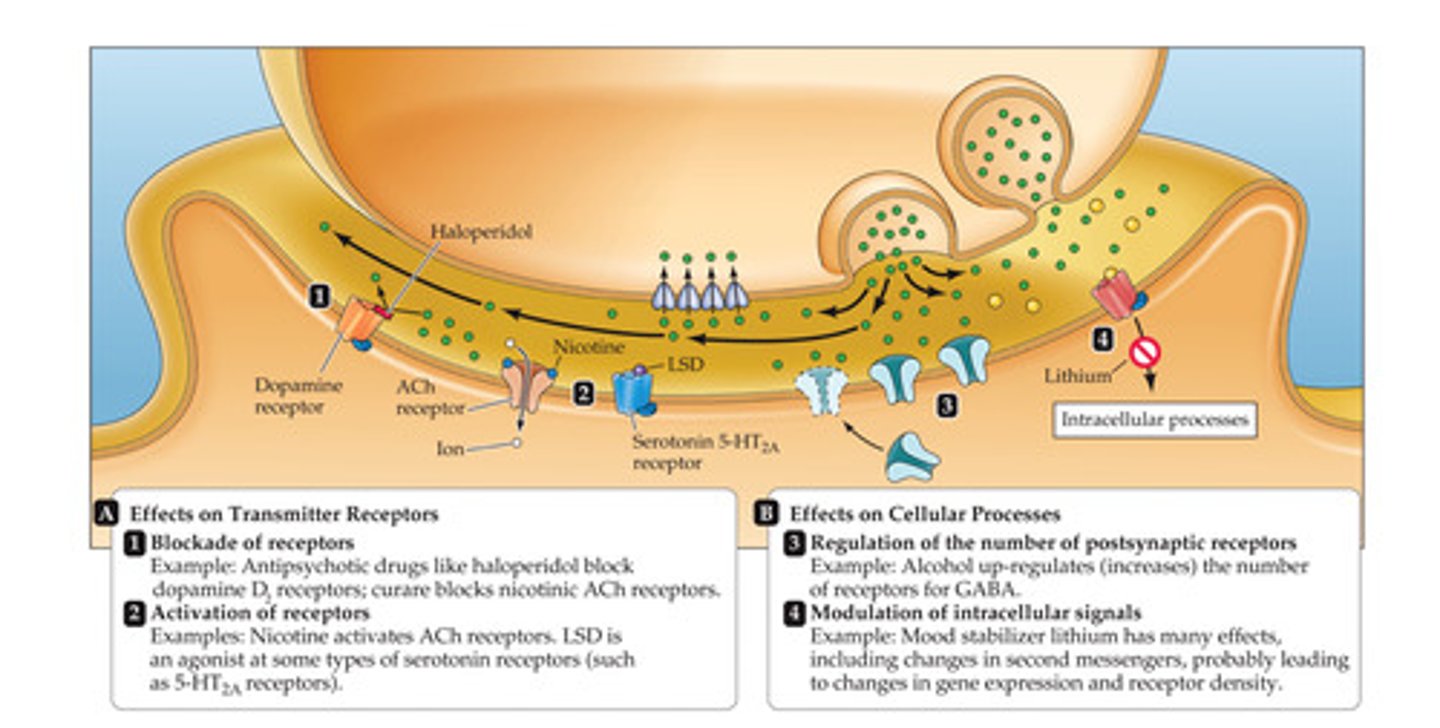
Presynaptic Drugs (Neurotransmitter Release)
1. Voltage-gated Na+ channel blockers
2. Voltage-gated Ca2+ channel blockers
3. Autoreceptor modulators
-Autoreceptor= receptors on the presynaptic neuron
4. Other modulators of neurotransmitter exocytosis
Drugs in the Synaptic Cleft (Neurotransmitter Clearance)
Reuptake inhibitors and enzymatic inhibitors
Natural selection
Adaptations are selected for and ultimate predominate within a population
Adaptations
Traits increasing the likelihood of reproduction
Ecological niches
Set of environmental opportunities and pressures
Gregor Mendel's (genetics) was linked to Hugo de Vries (evolution)
Genetic mutations are the substrate of evolution on which natural selection acts
The survival of an organism ____________ (increases/decreases) chances that genes are passed to offspring
Increases
Divergent evolution
Accumulation of trait differences between closely relate species, sometimes leading to speciation
Speciation
Formation of new species
Homology
Structural/functional/behavioral resemblance based on common ancestry
Convergent evolution
Independent evolution of similar traits in distantly related species
Homoplasy
Structural/functional/behavioral resemblance based on convergent evolution
Taxonomy
The classification of organisms
Phylogeny
The evolutionary history of a group of organisms
Why can we study other animals in neuroscience?
Evolutionary conservation of neuroanatomy, neurophysiology, and/or behavior
What is generally conserved and what differs across the animal kingdom?
Generally conserved:
1. cellular neurobiology
2. neurophysiology
3. principles of behavior
Differs:
1. Gross neuroanatomy
Common features (homologies) across vertebrate brains
-Approx. bilateral symmetry
-Spinal segmentation
-Hierarchical control
-CNS/PNS
-Functional specialization
Evolution of the Vertebrate Brain Relative Size Differences
Relative sizes, proportions, and locations of brain structures
--> modified based on adaptation to unique ecological niches
Encephalization Quotient (EQ)
The extent of deviation from the linear brain-to-body trend
Hominin
A subgroup of hominids (apes) including modern humans and our ancestors
Where was the largest increase in the brain?
Cerebral cortex (specifically the frontal lobe)
*Side note: the brain size tripled over the last 1.5 mil years*
Downsides of having a bigger brain
1. Long gestation time and difficult birthing
2. Brain growth continues for years after birth, requiring prolonged dependence on parents
3. High metabolic cost
4. Complex genes vulnerable to mutation
Brain regions that fully develop later in life....
1. Tend to have expanded most dramatically over evolution
2. Tend to serve more complex functions
Why did Hominin brain size explode?
-Environmental Models
-Social Models
-Cultural Models
Environmental Models (relative to brain size)
Physical challenges in the environment selected for larger brains
- Dietary factors
- Ecological factors
Social Models (relative to brain size)
Cooperative or competitive interactions selected for larger brains
-Hunting/gathering prowess
-Sexual selection
Sexual selection
A form of natural selection in which members of one sex favor specific heritable traits in the other sex during mating behavior
Cultural Models (relative to brain size)
Hominins able to retain and teach accumulated knowledge were more likely to reproduce
Evolutionary Psychology
A research field that studies how natural selection has shaped the behavior of humans and other animals
3 layers of the human embryo during gastrulation (approx. 14-21 days after conception)
1. Ectoderm
2. Mesoderm
3. Endoderm
*Side note- nervous system is one of the first organ systems to develop (day 18-19)*
The ________ induces formation of the neural plate from ectoderm
Notochord
(Day 18-19)
Neurulation- Formation of the Neural Tube
-Neural plate gives rise to neural tube (CNS) and neural crest (PNS+) (days 21-28)
-Rostral and caudal neuropores close, giving rise to the early brain and spinal cord (days 24-25 and 25-26)
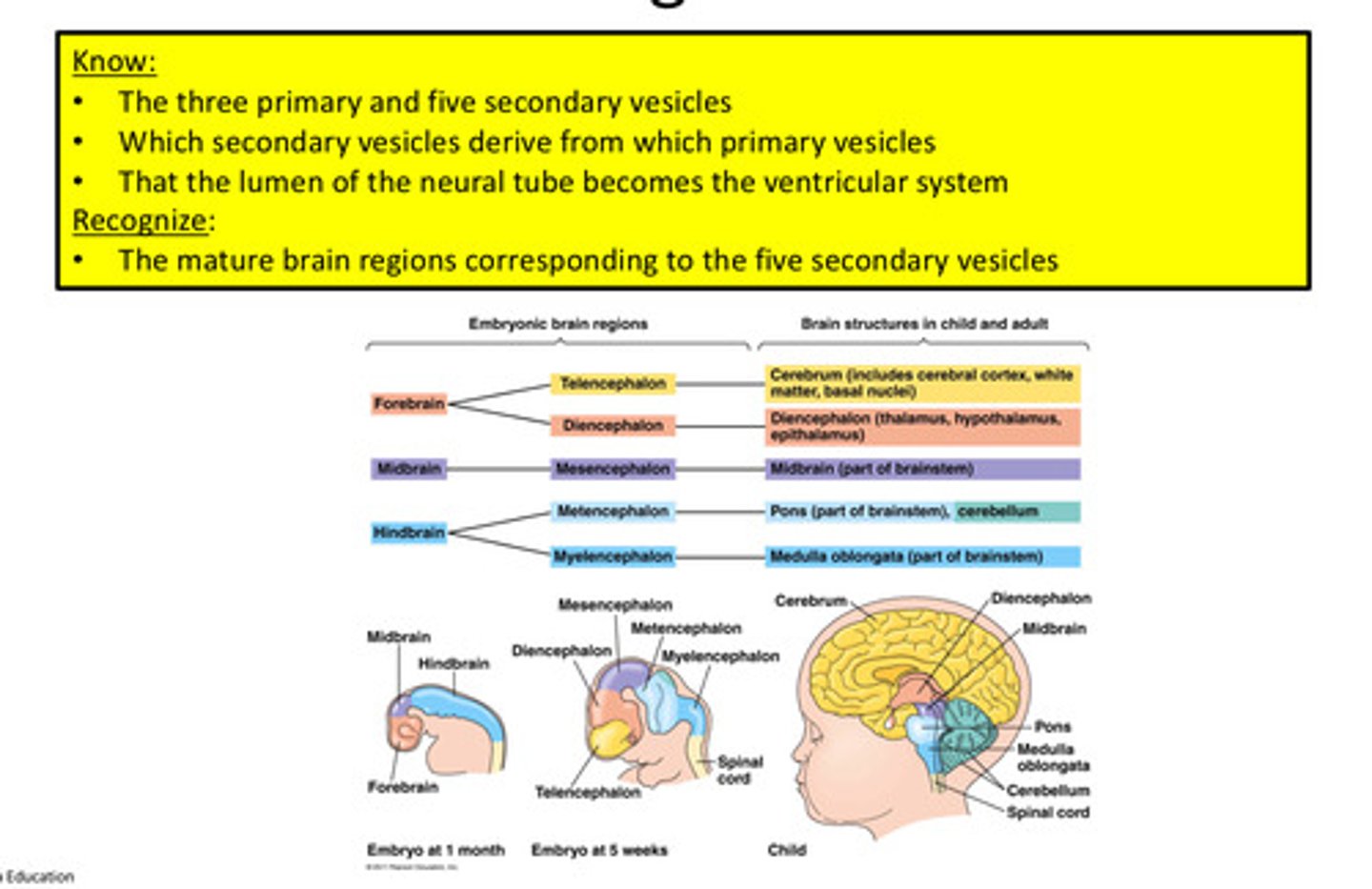
Subdivisions of the Neural Tube-Primary Vesicles (3)
1. Prosencephalon (forebrain)
2. Mesencephalon (midbrain)
3. Rhombencephalon (hindbrain)
"Probe Me Right"
Subdivisions of the Neural Tube-Secondary Vesicles (5)
1. Telencephalon: Cerebrum (cerebral hemispheres)
2. Diencephalon: Thalamus + Hypothalamus
3. Mesencephalon: Midbrain
4. Metencephalon: Pons + Cerebellum
5. Myelencephalon: Medulla
"Tell Di: Mes Met My!"
Which secondary vesicles derive from which primary vesicles
1. Forebrain---> Telencephalon, Diencephalon
2. Midbrain---> Mesencephalon
3. Hindbrain---> Metencephalon, Myelencephalon
Neural tube defects are often associated with
Maternal folic acid insufficiency
Anencephaly
-Failure of rostral neuropore closure
-Partial of complete absence of brain, cranial defects
-Most fetuses do not survive to term
Spina Bifida
-Failure of caudal neuropore closure
-Can be largely asymptomatic of involve a herniated sac
-Can often be surgically corrected
*Think Derek Shepards kid*
Fetal Alcohol Syndrome (FAS)
-Can result from maternal alcohol consumption
-Altered facial features, stunted growth
- Often intellectual disabilities, absent corpus callosum in severe instances
Autism Spectrum Disorder (AUS)
-Speculated to have both genetic and environmental causes
-Marked by impaired social interactions, language utilization, a narrow range of interests, and perseveration
Phenylketonuria (PKU)
-Recessive genetic disorder involving inability to metabolize the amino acid phenylalanine
- Susceptible to intellectual disability, especially before age 2, if phenylalanine is consumed in diet
Down Syndrome
-Trisomy 21
-Marked by intellectual impairment, stunted growth, various physical abnormalities
-Increased risk of epilepsy (seizure disorders) and Alzheimer's disease
Stages of Cellular Neurodevelopment
1. Neurogenesis
2. Cell migration
3. Differentiation
4. Synaptogenesis
5. Apoptosis
6. Synaptic remodeling
Neurogenesis
Stage 1 of cellular neurodevelopment
-Neural stem cells divide via mitosis to form neurons and glia
-Majority of neurons formed by birth, but limited adult neurogenesis