Oxidation and reductio and half equations
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
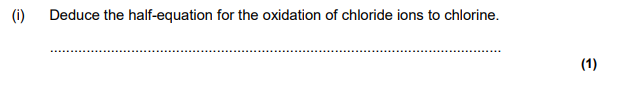


+7


what does manganese(II) mean?

Explain why chlorine has a lower boiling point than bromine. (2 marks)
Chlorine is smaller than bromine
Cl2 has weaker / fewer / less (VdW) intermolecular forces / forces between molecules
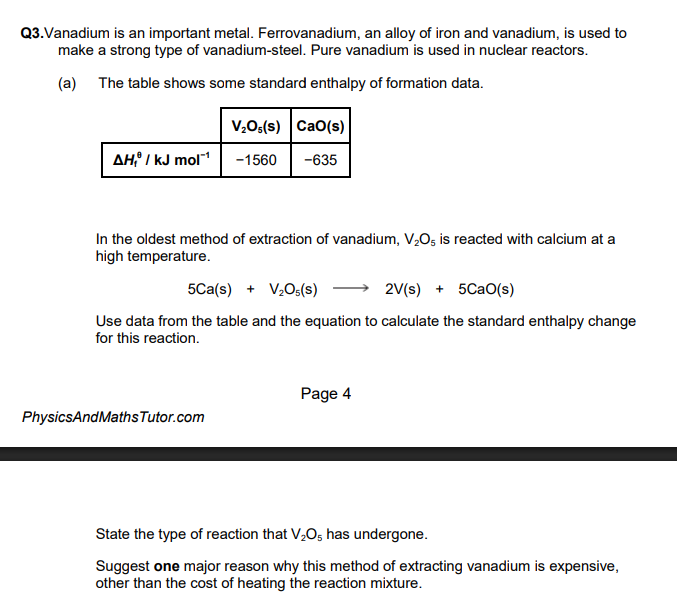
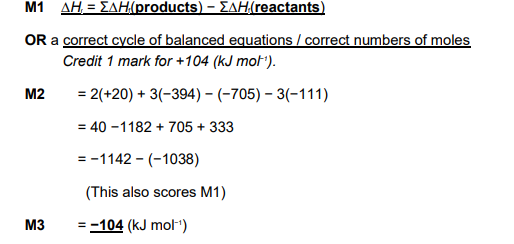
ΔH = ΣΔHf (products) − ΣΔHf (reactants)
= 5(−635) − (−1560)
= − 3175 + 1560
= − 1615 (kJ mol−1)
this is a reduction reaction
calcium is expensive to extract



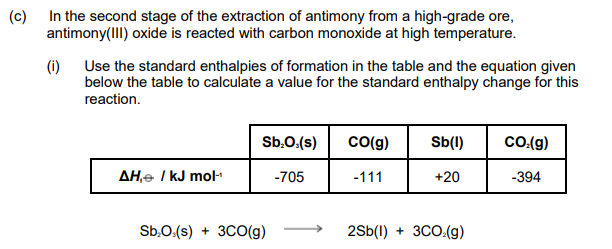
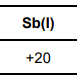
Suggest why the value for the standard enthalpy of formation of liquid antimony, given in the table above, is not zero.
Sb is not in its standard state
Or Standard state (for Sb) is solid / (s)

State the type of reaction that antimony(III) oxide has undergone in this reaction.
Reduction (+3 to 0)
When asked these sorts of questions on a reaction being high or low cost what should you look out for?
how many steps it takes
how expensive the materials/ catalysts are
How much energy it requires

It’s D. because I- has a free electron that is further away from the nucleus than Cl- so it reduces easier.