Shapes of Covalent Molecules
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
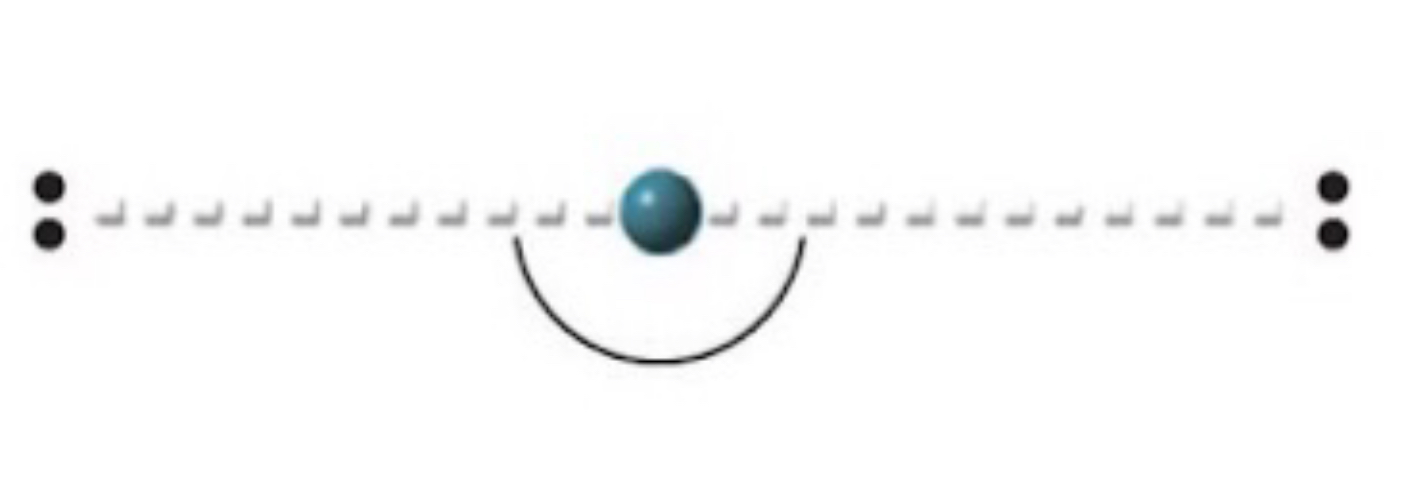
Name, electron groups and predicted bond angles?
Linear, 2 and 180 degrees
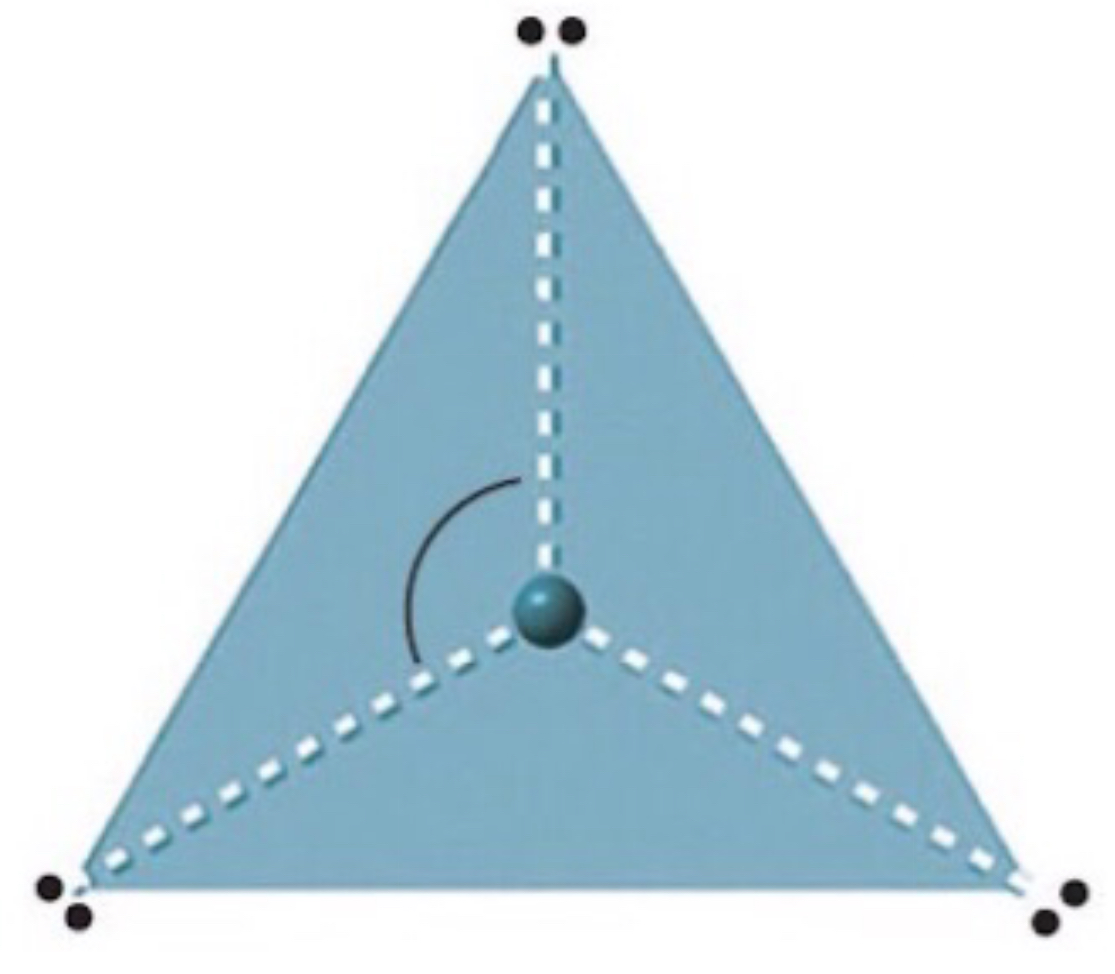
Name, electron groups and predicted bond angles?
Trigonal planar, 3 and 120 degrees
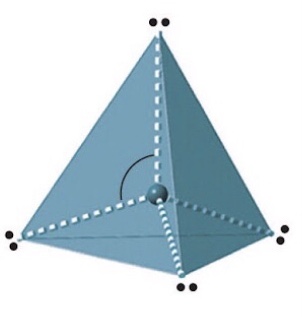
Name, electron groups and predicted bond angles?
Tetrahedral, 4 and 109.5 degrees
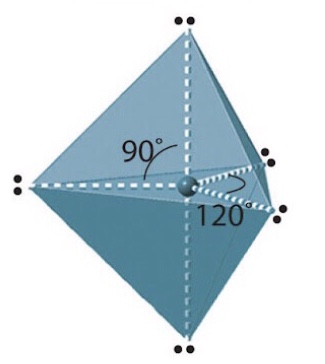
Name, electron groups and predicted bond angles?
Trigonal bipyramidal, 5 and 90 or 120 degrees
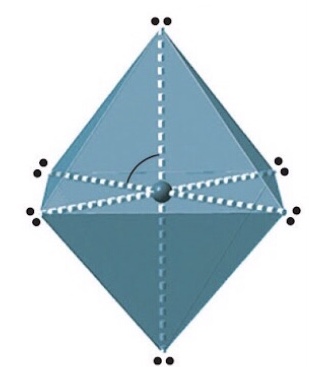
Name, electron groups and predicted bond angles?
Octahedral, 6 and 90 degrees
What does VESPR stand for?
Valence Shell Electron Pair Repulsion Theory
What does the VESPR Theory state?
That the shape of a molecule depends on the total number of pairs of electrons in the outer energy level of the central atom (electron pairs repel each other so they arrange themselves so they are as far away as possible from each other)
Why do lone pairs repel more strongly then bonding pairs?
They are closer to the nucleus so they distort the molecules shape because they reduce the bonding angle by 2.5 degrees for every lone pair.
Name the 2 covalent molecules with lone pairs
Pyramidal and v-shaped
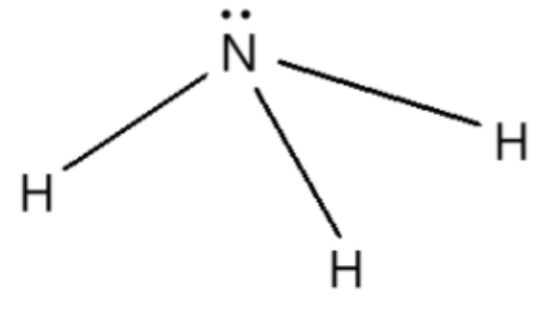
Name, electron groups, and predicted bond angles?
Expected tetrahedral because of 4 electron groups but there is a lone pair so predicted bond angle is 109.5-2.5= 107 degrees and pyramidal is the correct name.
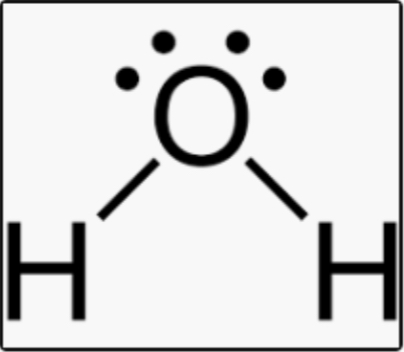
Name, electron groups, and predicted bond angles?