Cape Bio Water detailed
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
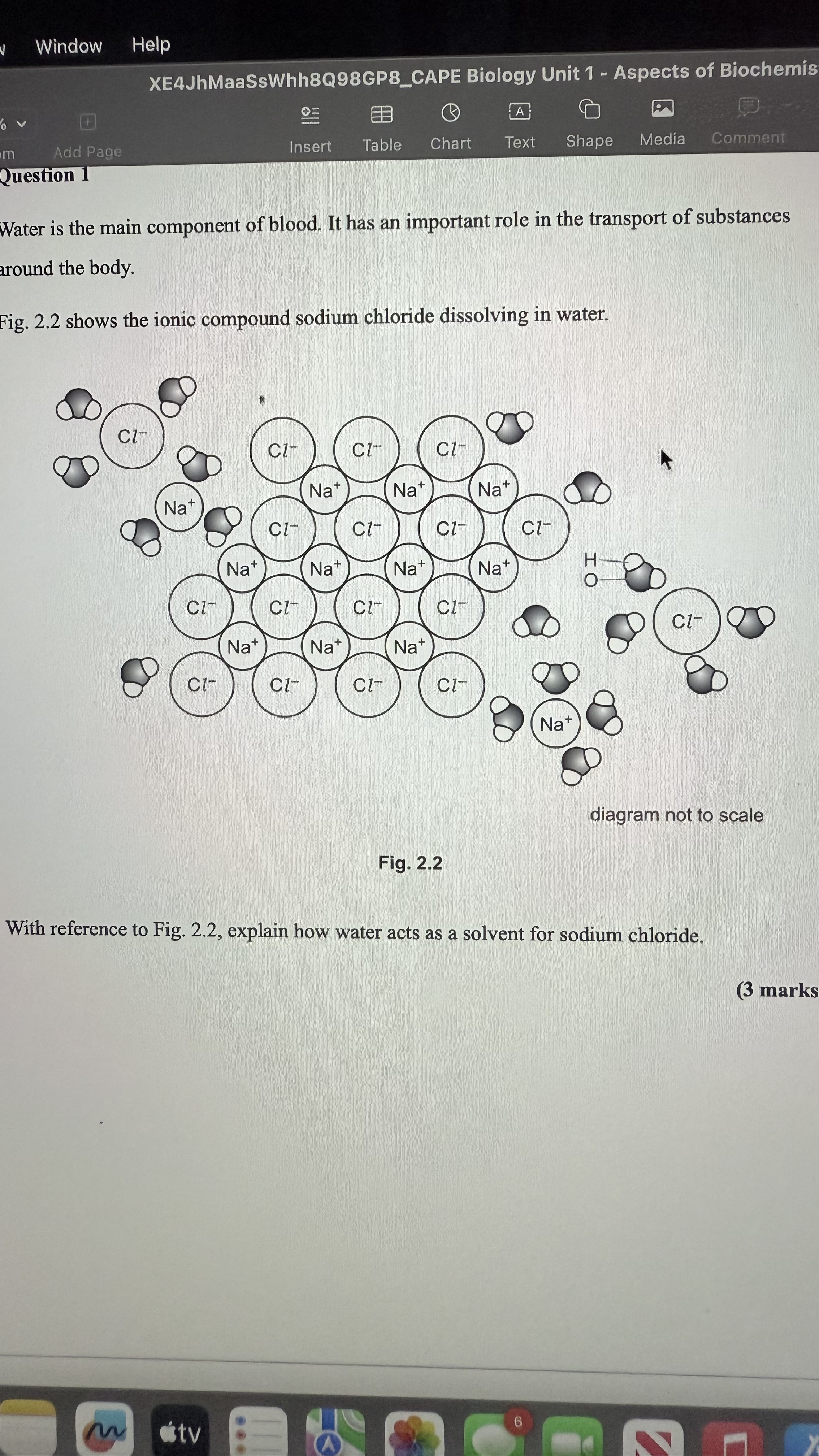
Explain how water acts as a solvent for sodium chloride.
The oxygen atom in water is attracted to the Na+ ion. The hydrogen atom in water is attracted to the Cl- ion.
The ionic bonds between the Na+ and Cl- ions are broken. Each ion becomes surrounded by a cloud of water molecules which keeps the ions apart. The ions are now in solution.
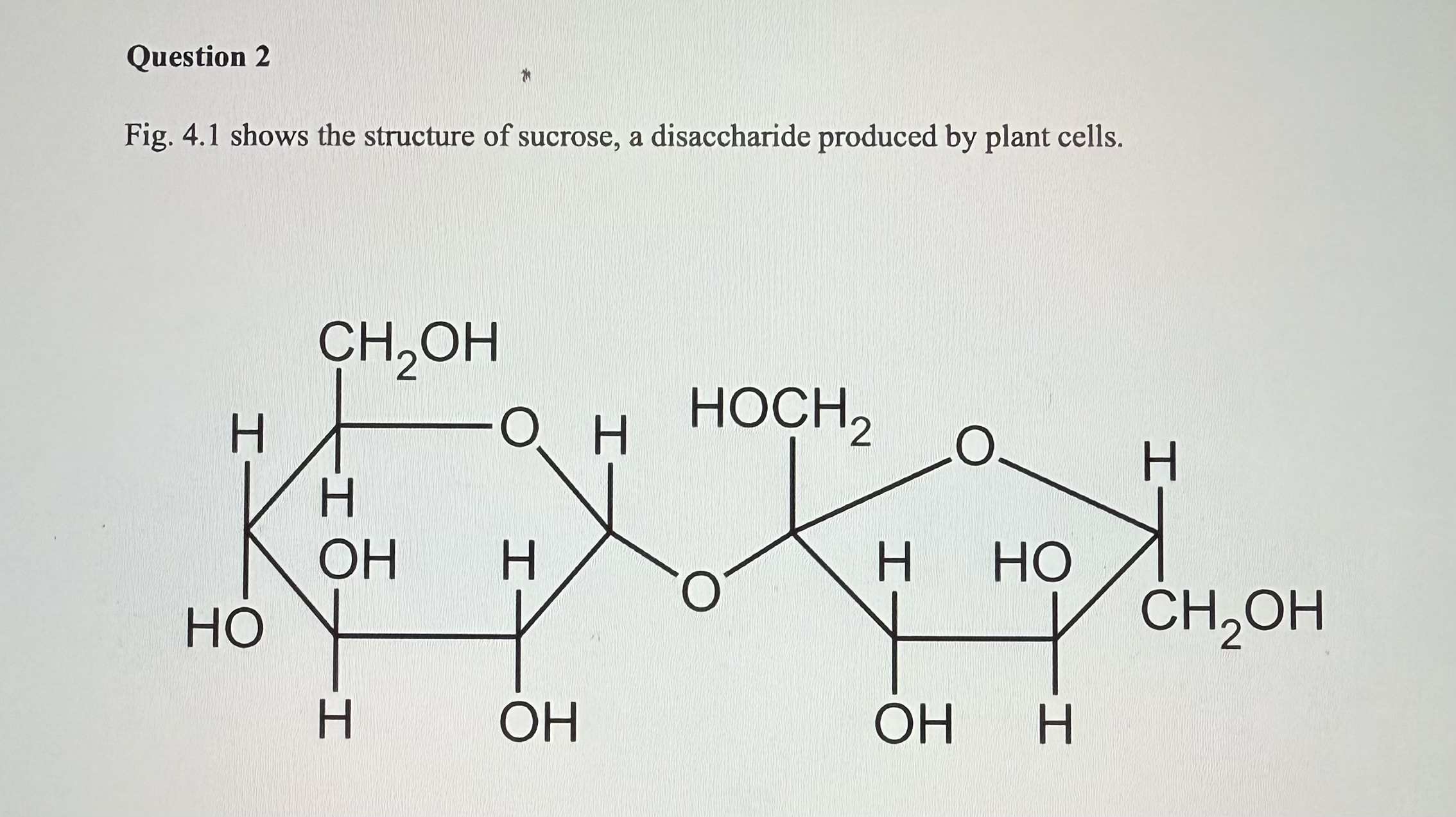
The structure attached by image is sucrose, a disaccharide produced by plant cells.
Name the covalent bonds between that joins the two monomers in sucrose
A Glycosidic Bond
Why type of bond does the atoms in water have?
A covalent bond
What is a hydrogen bond
The positively charged atoms in one molecule are attracted to the negatively charged oxygen atoms in other molecules
For a single water molecule in ice what is the maximum number of hydrogen bonds
4
Water description in biochemistry
Water is the most abundant molecule in our bodies. Water is a major component of all cells often making up between 70-90% of the cells mass
What is the specific capacity of water
4.2 j g-1 •C-1
How many joules of energy does it take to heat 1 gram of water by 1•C
4.2 j
Name the properties of water
High specific heat capacity
High Latent heat of vaporisation
High Latent heat of fusion
Good solvent
Density and viscosity
High Cohesion
Incompressible
Reactivity
What does a water molecule consist of
Two hydrogen atoms covalently bonded to one oxygen atom
Define solvent
Able to dissolve other substances
Define solute
The solute is the substance being dissolved
Define insoluble
Unable to be dissolved
Define dipolar
A molecule that has a separation of positive and negative charges, creating two poles, a positive and negative end
Define Anabolism
The reaction that build up larger biological molecules from smaller ones, they are known as anabolic reactions
Define catabolism
The reactions that break down large biological molecules into smaller ones they are known as catabolic reactions