Shapes of Molecules - VSEPR Theory
1/12
Earn XP
Description and Tags
This set of flashcards is focused on the key vocabulary and concepts from the VSEPR Theory and shapes of molecules.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
VSEPR Theory
1. The electrons in an atom’s valence shell (outer level) are responsible for chemical
bonding.
2. Electron pairs will repel each other so that they are as far apart as possible.
3. Lone pairs of electrons are more repulsive than shared pairs.
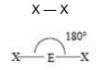
Linear
2 SA
0 LP
180 degrees
Linear II
2 SA
3 LP
180 degrees
Angular/Bent
2 SA
1 or 2 LP
104.5 degree bond angle
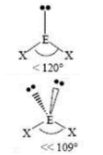
Trigonal Planar
3 SA
0 LP
120 degrees bond angle
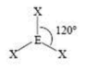
Trigonal Pyramidal
3 SA
1 LP
107 degrees bond angle
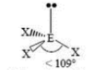
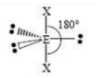
T-shaped
3 SA
2 LP
180 degrees & 90 degrees bond angles
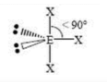
Tetrahedral
4 SA
0 LP
109.5 degrees bond angle
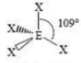
Seesaw
4 SA
1 LP
180 degrees & 90 degrees bond angles
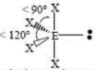
Square Planar
4 SA
2 LP
90 degrees bond angle
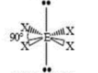
Trigonal Bipyramidal
5 SA
0 LP
120 degrees & 90 degrees bond angles
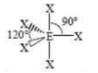
Square-based Pyramid
5 SA
1 LP
90 degrees bond angles
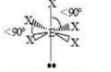
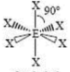
Octahedral
6 SA
0 LP
90 degrees bond angles