Bio Exam 2025 - Short Answer and MC
1/98
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
99 Terms
Explain passive transport by osmosis (Unit 1 - Osmosis)
The movement (diffusion) of water molecules across a semipermeable membrane is called osmosis.
Water moves along its concentration gradient until concentrations on each side of the membrane are equal.
Water moves because the membrane is impermeable to the solute and the solute concentrations may differ on either side of the membrane.
Diffuses from an area of lower solute concentration (high water concentration) to an area of greater solute concentration (low water concentration)
Occurs when there is an imbalance of solutes outside a cell vs inside a cell
What causes cells to swell or shrink? (Unit 1 - Osmosis)
In living cells, the inward of outward movement of water by osmosis develops forces that can cause cells to swell or shrink.
Two solutions with equal solute concentrations are isotonic.
(iso = “equal”) – Normal looking cell
If the concentration is unequal, the solution with the higher solute concentration is hypertonic. (hyper = “more than”) – shriveled cell
The solution with the lower solute concentration is hypotonic.
(hypo = “less than”) – Swollen cell
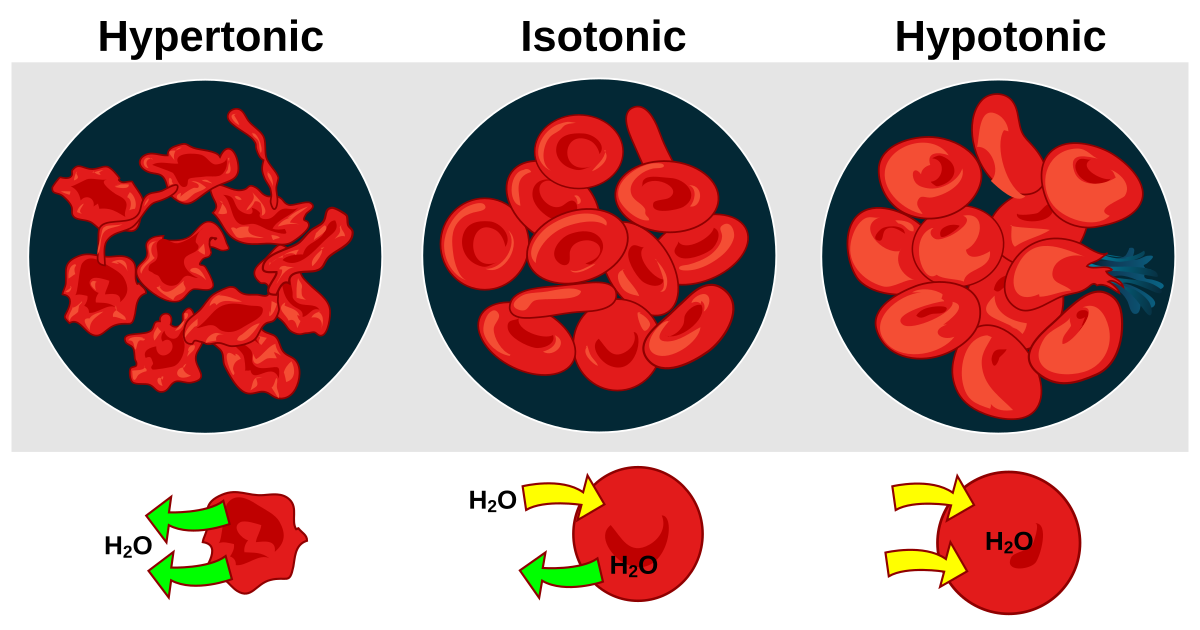
Explain how animal and plant cells behave differently when placed in hypotonic or hypertonic solutions. (Unit 1 - Osmosis)
Due to differences in structure, animal and plant cells behave
differently when placed in hypotonic or hypertonic solutions.
Arrows indicate the movement of water molecules.
Explain the structure and overall functions of carbs (Unit 1 - Macromolecules)
Structure:
contain only C, H, and O in ratio of 1:2:1
contain many OH (hydroxyl groups - very soluble in water)
contain one aldehyde or ketone
Overall functions:
Energy source – immediate energy & energy storage
Structural support (plants – cell wall)
Cell-to-cell communication/signalling – when attached to the cell membrane
Forms of carbs and their properties (a) (Unit 1 - Macromolecules)
Monosaccharides:
Simple sugars – the simplest type of carbohydrate (building blocks of carbohydrates) 3 -7 C atoms
When formed in water monosaccharides with five or more carbon atoms form a ring – reaction between functional groups (carbonyl and hydroxyl)
Monosaccharides - properties:
Many polar functional groups (-OH) = hydrophilic – highly soluble in water
Sweet taste – example: honey
Forms of carbs and their properties (b) (Unit 1 - Macromolecules)
Disaccharides:
Two monosaccharides linked together (dehydration reaction)
Ex. sucrose, maltose, lactose
Glycosidic bond - bond between two monosaccharides a
Forms of carbs and their properties (c) (Unit 1 - Macromolecules)
Complex carbs: polysaccharides
Complex carb: a molecule that is composed of hundreds to thousands of monosaccharides linked together; an essential part of nutrition and a valuable energy source.
Important for energy storage in cells (starch and glycogen)
Important for structural support (cellulose and chitin)
Many monosaccharides (long polymer – dehydration reactions) Insoluble in water
Polymerization - process in which small subunits (monomers) are linked together to form a large molecule (polymer) – through dehydration reaction
Types of Polysaccharides:
Starch
Cellulose
Glycogen
Starch (Unit 1 - Macromolecules)
amylose - ~ 100 glucose in a long, unbranched chain
amylopectin - a long chain of glucose with branched amylose chains
Starchy foods are an essential part of a balanced diet
Provide: energy, fiber and a sense of fullness
The body breaks down starch molecules into glucose, which is the body’s primary fuel source
Cellulose (Unit 1 - Macromolecules)
a long chain of glucose (100 - 1000) and no side chains
contains alternating glucose molecules
plant cell walls (fibres cannot be digested - since no enzymes)
Glycogen (Unit 1 - Macromolecules)
Form of energy storage in the human body – energy reserve
Stored in animal liver and muscle
A long, branched chain for the storage of glucose molecules
Many short branches
Lipids (Unit 1 - Macromolecules)
non-polar molecule that is made up of mostly C & H
Not polymers of defined monomeric subunits
Categories of lipids:
Fats
Phospholipids
Steroids
Waxes
Fats structure and function (Unit 1 - Macromolecules)
Structure:
a fat is made of 2 subunits combined by dehydration
Glycerol - a 3 C alcohol (each carbon is attached to one OH group) each carbon may be bonded to a fatty acid
Fatty Acid - a long, hydrocarbon chain ending with a carboxyl group # of C are even (4 - 24) but most common 16 or 18
One to three fatty acid chains are jointed to a single glycerol molecule
Dehydration reaction: - OH on glycerol and carboxyl on fatty acids
Triglycerides – most well-known fats (three fatty acid chains)
Function:
good source of energy due to the presence of many CH bonds (energy storage)
not soluble in water due lack of OH groups (hydrophobic)
Structural (cell membrane)
Some serve as hormones Insulators, cushion for vital organs
Forms of fats and their properties (a) (Unit 1 - Macromolecules)
Saturated - all internal C atoms are bonded to two H atoms
single bonds only
animal fat (hard fat) – e.g., butter, packed close together
Forms of carbs and their properties (b) (Unit 1 - Macromolecules)
Unsaturated - all internal C atoms do not bond to two H atoms
one double bond is present (creates kink and bends) - e.g., olive oil
Forms of carbs and their properties (c) (Unit 1 - Macromolecules)
Polyunsaturated - more than one internal C atom is missing H atoms
more than one double bond is present in C chain
tend to be fluids
oils are liquid fats (plant fats)
Phospholipid (Unit 1 - Macromolecules)
main component of the cell membrane
Glycerol makes up the backbone of phospholipids
Made up of a glycerol molecule + 2 F.A. + phosphate group (highly polar) + “R” group (another polar or charged unit)
Membrane fluidity of saturated and unsaturated fatty acids (Unit 1 - Macromolecules)
Saturated fatty acids:
No double bonds = relatively straight
At cooler temp. the straight tails can pack tight, making a fairly rigid membrane
Unsaturated fatty acids:
Double bond= bent
Cannot pack together as tightly, at cooler temp. membrane will stay fluid
Explain what factors affect enzyme activity (Unit 1 - Enzymes)
Enzyme activity is affected by any change in conditions that alters the 3D shape.
Temperature
pH
Enzymes affected by temp (Unit 1 - Enzymes)
Affects chemical reactions:
As the temperature rises, the rate of a chemical reaction usually increases
Reflects increase in the kinetic motion of the molecules
More frequent and stronger collisions
Affects all proteins, including enzymes:
As the temperature rises, the kinetic motion of the amino acid chains of an enzyme increases
Strength and frequency of collisions between the enzyme molecules and surrounding molecules increases
Enzymes have a max. activity within a narrow temperature range
Most enzymes peak activity is around 40 degrees
Above 40 degrees the enzyme begins to denature (unravel) and lose its ability to function
There are exceptions – E.g., fish in Antarctica (enzymes active near 0 degrees)
Enzymes effected by pH (Unit 1 - Enzymes)
Each enzyme has an optimal pH where it operates at its highest efficiency
Any changes from its optimal value decreases the rate of reaction
Most enzymes have a pH optimum that is near the pH of their cellular content (most enzymes 🡪 pH 7)
Enzymes that are secreted from cells have more variable pH optimal – E.g., Pepsin (optimal pH 1.5)
The more the pH deviates from the optimal value, the more extreme the effects are on the structure and function of the active site of the enzyme
Pepsin is a protein digesting enzyme that is secreted into the stomach. The pH of 1.5 is close to the acidity content of the stomach
Oxidative phosphorylation (unit 2 - ETC)
Oxidative phosphorylation is a process that couples the oxidation of NADH and FADH2 by the electron transport chain with the synthesis of ATP in chemiosmosis.
Oxygen acts as an electron acceptor, is converted to water, and aids in the transfer of energy from the electron carriers to ATP.
Electron transport chain (Unit 2 - ETC)
The electron transport chain is a series of electron carriers and proteins that are embedded in the inner membrane of a mitochondrion.
1. Electrons from FADH2 and NADH are donated here, providing the energy for oxidative phosphorylation.
2. NADH and FADH2 both pass two electrons into the chain, one at a time. One hydrogen ion from NADH and two from FADH2 are released and remain in solution in the matrix.
3. The electrons pass through three major complexes that use the energy from the electrons to actively pump hydrogen ions out of the matrix and into the intermembrane space, thus creating a hydrogen ion gradient. FADH2 skips the first complex, so only a total of four hydrogen ions are pumped in the case of FADH2, instead of six as by NADH.
4.The final electron acceptor is oxygen, which also combines with two hydrogen ions to produce water.
Chemiosmosis
Chemiosmosis is a process that uses the energy in a hydrogen ion gradient across the inner mitochondrial membrane to drive the production of ATP.
1.The hydrogen ions are restricted from flowing back into the matrix. The electrical potential energy is converted into chemical potential energy when the hydrogen ions flow through complexes called ATP synthases by facilitated diffusion.
2.In the final step of chemiosmosis, ATP is produced from ADP and Pi by the energy generated in the ATP synthases.
Two stages of photosynthesis (Unit 2 - photosynthesis)
Light-dependent reactions: light energy is trapped and used to generate two high-energy compounds (ATP and NADPH)
Light-independent reactions (Calvin Cycle): does not require light, uses ATP and NADPH to make a high-energy organic molecule
The absorption of light (Unit 2 - Photosynthesis)
Cyclic electron transport (cyclic photophosphorylation) (Unit 2 - Photosynthesis)
Photosystem I functions independently of photosystem II
Energy absorbed from light is converted into ATP without the oxidation of water or reduction of NADP+ to NADPH
Light energy captured in this cycle is used to drive phosphorylation of ADP to ATP
Cyclic electron transport plays an important role in overall photosynthesis. The reduction of carbon dioxide by the Calvin cycle requires more ATP than NADPH, and the additional ATP molecules are provided by cyclic electron transport.
Other energy-requiring reactions in the chloroplast are also dependent on ATP produced by the cyclic pathway.
When and why cyclic electron transport occurs (Unit 2 - photosynthesis)
ATP/NADPH Balance:
Cyclic electron transport often occurs when there is a higher demand for ATP relative to NADPH in the chloroplast. For example, during times when the Calvin cycle requires more ATP for carbon fixation and regeneration of ribulose bisphosphate (RuBP), cyclic electron transport can help meet this demand without producing additional NADPH.
Low Light Conditions:
Under low light conditions, the efficiency of non-cyclic electron transport may decrease. In these situations, cyclic electron transport can help sustain ATP production, allowing the plant to continue with metabolic processes that require energy.
High NADPH Levels:
If the levels of NADPH are already high due to previous light reactions (non-cyclic electron transport), there may be less need for NADPH production. In this case, the plant may favor cyclic electron transport to generate more ATP without increasing NADPH levels further.
Stress Conditions:
During stress conditions (e.g., drought, high salinity), plants may utilize cyclic electron transport to optimize energy production while conserving resources.
Light-independent reactions: the Calvin cycle (Unit 2 - photosynthesis)
Occurs in stroma
11 enzyme-catalyzed reactions which use NADPH to reduce CO2 into sugar
Endergonic (energy supplied by ATP)
3 phases
C3 Photosynthesis – process of converting CO2 to G3P using only the Calvin cycle
DNA replication: step 1 strand separation (Unit 3 - DNA replication)
Enzyme called helicase binds to these origins and begins to unwind the two strands of DNA by breaking H-bonds between complementary base pairs
Forms a replication fork – point of separation of the two parent DNA strands during replication
Topoisomerase: step 1 (Unit 3 - DNA replication)
Topoisomerases – enzymes that relieve tension caused by the unwinding of DNA; cuts one or two of the DNA strands, allow strands to untwist, then rejoin the cut strand(s)
SSBs: step 1 (Unit 3 - DNA replication)
After the two strands are separated, they have a tendency to rejoin, because they are complementary
Single-strand binding protein (SSB) - replication enzyme that prevents parent DNA strands from annealing to each other once they have been separated by helicase
Building complementary stands: step 2 (Unit 3 - DNA replication)
Enzyme DNA polymerase III add nucleotides to the 3’ end of the new developing strand while moving along and “reading” the template strand in the 3’ to 5’ direction
Can only add nucleotides to the 3’ end
Therefore, the new strand is assembled in the 5’ to 3’ direction
Once the strands have been separated, short fragments of nucleotide sequences that are used to start or “prime” DNA replication are called RNA primers
RNA primer binds to the 3’ end of the strand produced by the enzyme RNA primase
DNA polymerase III begins adding DNA nucleotides to the RNA primer one at a time to create a strand of DNA that is complementary to parent strand
Leading strand can replicate continuously, lagging strand must replicate in short DNA fragments called Okazaki fragments (between 100-200 nucleotides long)
DNA polymerase III can only add nucleotides to the 3’ end of a strand
As each fragment extends in the 5’ to 3’ direction, it eventually runs into the RNA primer attached to the Okazaki fragment ahead of it
DNA polymerase I – enzyme that replaces RNA primer, replaces RNA nucleotides with DNA nucleotides
Once the primer is replaced, the last nucleotide is linked to the Okazaki fragment in front of it by the formation of a phosphodiester bond – reaction catalyzed by DNA ligase
DNA ligase – enzyme that joins together the Okazaki fragments
Proofreading and Repairs: step 3 (Unit 3 - DNA replication)
DNA Polymerase III – as they assemble new DNA strands, they proofread and correct errors
Errors are usually base-pair mismatches
Example: If a thymine is added across from a cytosine, they cannot form hydrogen bonds, and the strand is unstable
DNA polymerase III backs up, replaces incorrect base and continues on
After a strand has been replicated, rare mismatching errors may still be present – average of one error for every million base pairs
DNA Polymerase II - enzyme that repairs damage to DNA
Mismatch base pairs distort shape of DNA, DNA polymerase II locates distortions and removes portion of strand
Gap filled in by DNA polymerase III/DNA ligase
Elongation (Unit 3 - transcription and translation)
RNA polymerase makes a complementary RNA copy in the 5’ to 3’ direction, using the 3’ to 5’ template DNA strand
Opposite strand not being copied is the coding strand – contains the same base-pair sequence as the new RNA molecule ( U 🡪 T)
RNA polymerase produces a precursor mRNA (pre-mRNA)
Translation: Nucleic Acid to Polypeptide (Unit 3 - transcription and translation)
tRNA
Small – 70 to 90 nucleotides long
Regions that base pair with themselves
Four double helical segments (cloverleaf pattern)
One tip has anticodon (3-nucleotide segment that pairs with a codon in an mRNA), other end carries amino acid that corresponds with anticodon
For example: a tRNA that is linked to serine (Ser) pairs with the codon 5’ – AGU – 3’ in mRNA. The anticodon of the tRNA that pairs with this codon is 3’ – UCA – 5’
Termination (Unit 3 - transcription and translation)
When the A site of a ribosome arrives at one of the stop codons (UAA, UAG, or UGA) on the mRNA
Roles of mRNA, tRNA, and rRNA in protein synthesis (Unit 3 - transcription and translation)
messenger RNA (mRNA) – RNA that contains the genetic information of a gene and carries it to the protein synthesis machinery; is translated by ribosomes into a protein
transfer RNA (tRNA) - carrier molecule that binds to a specific amino acid and adds the amino acid to the growing polypeptide chain
ribosomal RNA (rRNA) – an RNA molecule within the ribosome that bonds the correct amino acid to the polypeptide chain
Types of mutations (Unit 3 - transcription and translation)
The effects of glucose imbalance (Unit 4 - Diabetes)
Diabetes mellitus – a serious chronic condition that results when the pancreas does not make enough insulin, or the body does not respond properly to insulin; levels of blood glucose tend to rise sharply after meals (hyperglycemia – high blood sugar) and remain at significantly elevated levels
The effects of glucose imbalance - Hyperglycemia (Unit 4 - Diabetes)
Hyperglycemia has various short-term and long-term effects on the body.
Without insulin, cells remain relatively impermeable to glucose and cannot obtain enough from the blood. The individual experiences fatigue as the cells become starved for glucose. The body compensates to some degree by switching to protein and fat metabolism for energy.
Fats and proteins are less accessible, however, and more difficult than glucose to break down. Fat metabolism also releases ketones, such as acetone, as a toxic by-product, which can be smelled on the breath.
The effects of glucose imbalance - kidneys (Unit 4 - Diabetes)
The kidneys are incapable of reabsorbing all the glucose that is filtered through them from the blood, and so glucose is excreted in the urine.
Due to the concentration gradient in the kidneys, large volumes of water follow the glucose into the urine and get excreted. People with untreated diabetes experience low energy and great thirst and produce large volumes of glucose-rich urine.
In the long term, continued high levels of blood glucose can lead to blindness, kidney failure, nerve damage, and gangrene (a severe infection) in the limbs.
Action potential: step 1 (Unit 4 - Action potential)
An action potential is triggered when the threshold potential is reached.
Influx of Na+ from another nerve signal reaching the dendrites.
Action potential: step 2 (Unit 4 - Action potential)
Voltage-gated Na+ channels open when the threshold potential is reached (cell becomes less negative/more positive).
Na+ move down [gradient] and rush into the axon
Membrane is depolarized
membrane potential difference is now +40 mV. (more positive charges inside cell)
Action potential: step 3 (Unit 4 - Action potential)
Voltage-gated Na+ channels close due to change in membrane potential
Caused by Na+ moving into the cell
Voltage-gated K+ channels open at this voltage (+40mV)
K+ move down [gradient] and exit the axon (cell)
membrane becomes hyperpolarized to -90 mV
Due to K+ leaving
Action potential: step 4 (Unit 4 - Action potential)
Voltage-gated K+ channels close.
Na-K pump and naturally occurring diffusion (leaky membrane) restore resting membrane potential of -70 mV
The membrane is now repolarized once the Na-K pump restores pre-action potential concentrations
Parts of the nephron (Unit 4 - Urine formation in the nephron)
Explain the parts of the nephron (Unit 4 - Urine formation in the nephron)
Active transport across the membrane (MC)
The movement of substances across the membrane using energy from ATP (against concentration gradient using pumps)
Passive transport across the membrane (MC)
The movement of substances across the membrane without expending energy
Passive transport by diffusion
Diffusion is a type of passive transport. Diffusion through a permeable membrane moves a substance from an area of high concentration (extracellular fluid, in this case) down its concentration gradient (into the cytoplasm) to an area of low concentration.
The cell membrane is semi-permeable
Passive transport means that some substances do not require energy to move across the cell membrane because they move down their concentration gradients.
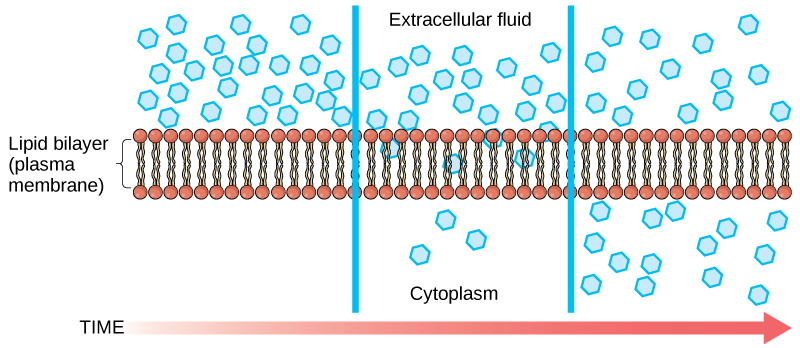
Diffusion (what is it and what is the rate affected by)
the net movement of ions or molecules from an area of higher concentration to an area of lower concentration. The rate of diffusion across a membrane is affected by:
• molecule size
• molecule or ion charge
• molecule polarity
• temperature and pressure (an increase in both increases the rate)
Hydroxyl (name of compounds, functional properties) (MC)
Name of compounds:
Alcohols
Functional properties:
Is polar as a result of the electronegative oxygen atom drawing electrons towards itself
Attracts water molecules, helping dissolve organic compounds such as sugars
Carbonyl (name of compounds, functional properties) (MC)
Name of compounds:
Ketones if the carbonyl group is within a carbon skeleton
Aldehydes if the carbonyl group is at the end of the carbon skeleton
Functional Properties:
A ketone and an aldehyde may be structural isomers with different properties
Carboxyl (name of compounds, functional properties) (MC)
Name of compounds:
Carboxylic acids
Functional Properties
Has acidic properties because it is a source of hydrogen ions
The covalent bond between oxygen and hydrogen is so polar that the hydrogen ions (H+) tend to dissociate reversibly
In cells, found in the ionic form, which is called a carboxylate group
Amino (name of compounds, functional properties) (MC)
Name of compounds:
Amine
Functional Properties
Acts as a base; can pick up a proton from the surrounding solution
Ionized, with a charge of 1+, under cellular conditions
Sulfhydryl (name of compounds, functional properties) (MC)
Name of compounds:
Thiols
Functional Properties:
Two sulfhydryl groups can interact to help stabilize protein structure
Phosphate (name of compounds, functional properties) (MC)
Name of compounds:
Organic phosphates
Functional Properties:
Makes the molecule of which is it a part of an anion (negatively charged ion)
Can transfer energy between organic molecules
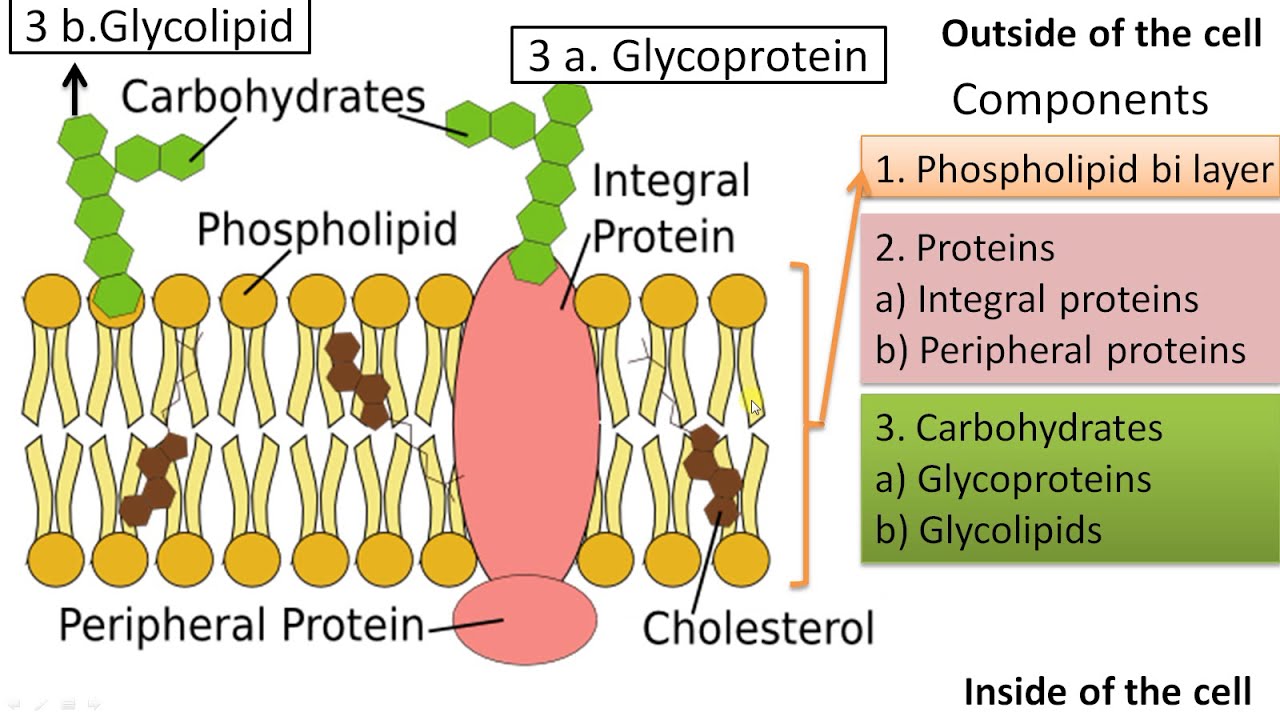
Fluid Mosaic Model (MC)
A fluid phospholipid bilayer with a mosaic of free-floating proteins embedded:
proteins in the bilayer have non-polar segments in contact with the non-polar interior of the bilayer
other proteins and molecules float in or on the bilayer
the two layers (leaflets) can slide across each other
individual phospholipids exchange places frequently
Phospholipid bilayer: Two layers of phospholipids arranged so their hydrophobic tails are projecting inwards, and their polar head groups project outwards
The cell membrane is a mosaic of components –phospholipids, cholesterol, proteins, carbohydrates – that move and float freely
Mosaic: made up of many different molecules (proteins, lipids, carbohydrates)
Dehydration reaction (MC)
dehydration reactions involve the formation of new bonds, requiring energy.
Hydrolysis reactions (MC)
hydrolysis reactions break bonds and release energy.
Function of enzymes (MC)
Enzymes are proteins that help speed up metabolism, or the chemical reactions in our bodies. They build some substances and break others down.
Inhabitation of enzymes (MC)
Inhibitors are molecules that interact with an enzyme and reduce the activity of the enzyme by interfering with its interaction with the substrate.
Competitive Inhibition (MC)
• A situation in which a competitor substance (inhibitor), that resembles the substrate, binds to a normal substrate binding site to block enzyme activity
• This blocks access to the substrate and slows the rate of reaction
• The inhibitor competes with the substrate for access to the active site on the enzyme
Examples: Cyanide, Carbon Monoxide
Non-competitive Inhibition (MC)
• A situation in which specific molecules bind to an enzyme at a site that is not the active site
• This changes the shape of the enzyme so that the substrate cannot bind to the active site and thus blocking enzyme activity
• Does not compete with substrate molecule
• Changes the shape of the active site*
Reversible vs. Irreversible Inhibition (MC)
• Inhibitors differ in how strongly they bind to enzymes
Reversible inhibition – binding of the inhibitor to the enzyme is weak and readily reversible
• Enzyme activity returns to normal following release of inhibitor
Irreversible inhibition – bind strongly to the enzyme through formation of covalent bonds (disable enzyme)
• Overcome only by the cell synthesizing more of that enzyme
Allosteric Control of Enzyme Activity (MC)
• Allosteric Site: a binding site on an enzyme (not its active site) that binds regulatory molecules
• Allosteric Regulation: the regulation of one site of a protein by binding to another site on the same protein
• Allosteric Inhibitor – a molecule that causes an enzyme to have a low affinity for a substrate, causing a release from the
active site
• Allosteric Activator – a molecule that causes an enzyme to have a high affinity for the substrates it binds
Redox reactions (MC)
reactions that involve the transfer of electrons from one species to another.
ATP (MC)
Adenosine triphosphate (ATP) is the source of energy for use and storage at the cellular level. The structure of ATP is a nucleoside triphosphate, consisting of a nitrogenous base (adenine), a ribose sugar, and three serially bonded phosphate groups.
Glycolysis (MC)
Location: Cytoplasm
Description: Glycolysis is the first step in the breakdown of glucose, converting one glucose molecule (C₆H₁₂O₆) into two molecules of pyruvate (C₃H₄O₃).
ATP Produced:
Theoretical ATP yield: 4 ATP (but 2 ATP are used for activation, so the net gain is 2 ATP).
Actual ATP yield: 2 ATP (net gain), plus 2 NADH molecules.
Electron/Proton Flow: No electron transport chain involvement, as glycolysis is an anaerobic process.
Final Products:
2 Pyruvate molecules
2 NADH molecules
2 ATP (net)
Pyruvate Oxidation (P.O.) (MC)
Location: Mitochondrial Matrix (after pyruvate enters mitochondria)
Description: Each pyruvate is decarboxylated (losing one carbon as CO₂) and combined with Coenzyme A to form Acetyl-CoA. NADH is generated in this process.
ATP Produced:
Theoretical ATP yield: No direct ATP production in this stage.
Actual ATP yield: 2 NADH molecules (1 per pyruvate molecule).
Electron/Proton Flow: NAD+ is reduced to NADH.
Final Products:
2 Acetyl-CoA (from 2 pyruvate molecules)
2 NADH
2 CO₂ (waste products)
Oxidative Phosphorylation (MC)
Location: Inner Mitochondrial Membrane
Description: This stage consists of the electron transport chain (ETC) and chemiosmosis, where electrons from NADH and FADH₂ pass through protein complexes, creating a proton gradient. The flow of protons through ATP synthase generates ATP.
ATP Produced:
Theoretical ATP yield: 3 ATP per NADH and 2 ATP per FADH₂.
Actual ATP yield: NADH (from Glycolysis, Pyruvate Oxidation, and Krebs Cycle) yields about 2.5 ATP each (total 10 NADH, resulting in ~25 ATP).
FADH₂ yields about 1.5 ATP each (total 2 FADH₂, resulting in ~3 ATP).
This leads to about 28 ATP from NADH and FADH₂.
Total ATP from Oxidative Phosphorylation (Chemiosmosis): ~28 ATP (with variations based on shuttle mechanisms for NADH from glycolysis).
Electron/Proton Flow:
Electrons from NADH and FADH₂ flow through the ETC complexes (I, III, IV).
Protons (H⁺) are pumped across the inner mitochondrial membrane, creating a proton gradient.
This proton gradient drives ATP production via ATP synthase.
Final Products:
6 H₂O (formed when electrons combine with oxygen)
~28 ATP (depending on the efficiency and shuttle mechanisms used)
Substrate level phosphorylation (MC)
• The formation of ATP by the direct transfer of a phosphate group from a substrate to ADP
Decarboxylation (MC)
• The formation of CO2 from the removal of a carboxyl group
Oxidative phosphorylation (MCO
• A process that forms ATP using energy transferred indirectly from a series of redox reactions (in aerobic respiration- NADH and FADH2 is the electron donor and oxygen is the final electron acceptor)
Chlorophyll a vs b (MC)
Chlorophyll a absorbs violet and orange light the most.
Chlorophyll b absorbs mostly blue and yellow light.
They both also absorb light of other wavelengths with less intensity.
Semiconservative replication (MC)
mechanism of DNA replication involving separating the two parent strands and building a new, complementary replacement strand for each.
Hershey & Chase, 1952 (MC)
investigated bacteriophages: viruses that infect bacteria
the bacteriophage was composed of only DNA and protein (inner nucleic acid core and outer protein coat called a capsid)
they wanted to determine which of these molecules is the genetic material that is injected into the bacteria
Bacteriophage DNA was labeled with radioactive phosphorus (32P)
Bacteriophage protein was labeled with radioactive sulfur (35S)
radioactive molecules were tracked
only the bacteriophage DNA (as indicated by the 32P) entered the bacteria and was used to produce more bacteriophage
Hersey-Chase experiment (MC)
DNA is the genetic material.
Recombinant DNA tech - Restriction endonucleases (MC)
The first step in the development of recombinant DNA technology was the characterization of restriction endonucleases—enzymes that cleave DNA at specific sequences. These enzymes were identified in bacteria, where they apparently provide a defense against the entry of foreign DNA (e.g., from a virus) into the cell.
Thyroxine (T4) (Production, acts where, function) (MC)
Production: Thyroid gland (specifically in the follicles of the thyroid)
Acts Where: Throughout the body, primarily in cells that regulate metabolism
Function:
T4 (Thyroxine) is a precursor to T3 (Triiodothyronine), the more active thyroid hormone.
It regulates metabolism by increasing the basal metabolic rate (BMR), promoting growth and development, and influencing body temperature.
Antidiuretic Hormone (ADH) (Production, acts where, function) (MC)
Production: Hypothalamus (stored and released by the posterior pituitary gland)
Acts Where: Kidneys (principal cells in the collecting ducts)
Function:
ADH helps regulate water balance in the body.
It increases water reabsorption by the kidneys, which reduces urine output and helps maintain blood volume and blood pressure.
Follicle-Stimulating Hormone (FSH) (Production, acts where, function) (MC)
Production: Anterior pituitary gland
Acts Where: Ovaries (females) and testes (males)
Function:
In females, FSH stimulates the growth and maturation of ovarian follicles and the production of estrogen.
In males, it stimulates the production of sperm (spermatogenesis) in the testes.
Epinephrine (Adrenaline) (Production, acts where, function) (MC)
Production: Adrenal medulla (part of the adrenal glands)
Acts Where: Various tissues (heart, lungs, muscles, liver)
Function:
Epinephrine is involved in the "fight or flight" response, increasing heart rate, blood pressure, blood sugar levels, and blood flow to muscles.
It also promotes the breakdown of glycogen into glucose in the liver and muscles (glycogenolysis).
Cortisol (Production, acts where, function) (MC)
Production: Adrenal cortex (zona fasciculata)
Acts Where: Various tissues (especially liver, muscle, and fat)
Function:
Cortisol is a stress hormone that helps regulate metabolism, the immune response, and inflammation.
It promotes gluconeogenesis (the formation of glucose from non-carbohydrate sources), suppresses inflammation, and modulates the body's response to stress.
Parathyroid Hormone (PTH) (Production, acts where, function) (MC)
Production: Parathyroid glands (typically four glands located behind the thyroid)
Acts Where: Bones, kidneys, and intestines
Function:
PTH increases blood calcium levels by stimulating osteoclasts to release calcium from bones.
It also enhances calcium reabsorption in the kidneys and increases the activation of vitamin D, which improves calcium absorption in the intestines.
Human Growth Hormone (hGH) (Production, acts where, function) (MC)
Production: Anterior pituitary gland
Acts Where: Liver, muscles, bones, and other tissues
Function:
hGH stimulates growth, cell reproduction, and regeneration.
It promotes the release of insulin-like growth factors (IGFs) from the liver, which aid in bone and tissue growth and development.
It also affects metabolism, increasing protein synthesis and fat breakdown.
Insulin (Production, acts where, function) (MC)
Production: Pancreas (beta cells in the islets of Langerhans)
Acts Where: Muscle, liver, and adipose tissue
Function:
Insulin lowers blood glucose levels by facilitating the uptake of glucose into cells for use as energy or storage as glycogen.
It also promotes fat storage and inhibits the breakdown of fat (lipolysis).
Glucagon (Production, acts where, function) (MC)
Production: Pancreas (alpha cells in the islets of Langerhans)
Acts Where: Liver and adipose tissue
Function:
Glucagon raises blood glucose levels by stimulating glycogen breakdown (glycogenolysis) and glucose production (gluconeogenesis) in the liver.
It also promotes the breakdown of fat in adipose tissue for energy use.
Calcitonin (Production, acts where, function) (MC)
Production: Thyroid gland (parafollicular cells, also known as C cells)
Acts Where: Bones and kidneys
Function:
Calcitonin lowers blood calcium levels by inhibiting osteoclasts (which break down bone) and increasing calcium excretion by the kidneys.
Glucocorticoids (e.g., cortisol) (Production, acts where, function) (MC)
Production: Adrenal cortex (zona fasciculata)
Acts Where: Various tissues (liver, muscles, fat)
Function:
Glucocorticoids regulate metabolism, immune response, and stress adaptation.
They increase blood glucose levels through gluconeogenesis, suppress inflammation, and help the body respond to stress.
Sympathetic nervous system (MC)
Nerve cells of the ANS that prepare the body for stress (fight or flight)
Neurotransmitter norepinephrine is released
Excitatory effect on its target muscles
Adrenal glands release epinephrine and norepinephrine
Activate stress response
Some areas of body are inhibited
Automatic system (MC)
Automatic/involuntary control
Nerves stimulate or inhibit glands/cardiac or smooth muscle
Controlled by the hypothalamus and medulla oblongata
Divided into sympathetic and parasympathetic nervous system
Somatic system (MC)
The part of the peripheral nervous system is responsible for voluntary movements and the transmission of sensory information to the central nervous system. It controls skeletal muscles and reflexes.
Sensory Neurons carry information from the external environment inward.
Motor neurons carry information to skeletal muscle.
Parasympathetic Nervous System (MC)
Nerve cells of the autonomic nervous system that return the body to normal levels after adjustments to stress
Restores and conserves energy
Rest and digest response: slows the heart rate, reduces blood pressure, promotes the digestion of food, and stimulates the reproductive organs by dilating blood vessels to the genitals
Central nervous system (MC)
consists of the brain and spinal cord; integrates and processes information sent by nerves
Peripheral Nervous System (MC)
Network of nerves that carry sensory messages to the central nervous system (CNS) and send information from the CNS to the muscles and glands
Central Nervous System (MC)
Consists of the brain and spinal cord; integrates and processes information sent by nerves
Negitive feedback loop example (MC)
An example is when the body reacts to becoming cold (shivering)
Sensor - skin
Control centre - brain
Effector - muscles (shivering)
Positive feedback loop example (MC)
An example is giving birth. When pushing the baby, a signal is sent to the brain to continue continuous contraction of the uterus.
Sensor - cervex
Control centre - brain
Effector - uterus