Chemistry R1.4 Entropy and Gibb's Energy
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 8:58 PM on 11/23/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
Entropy S
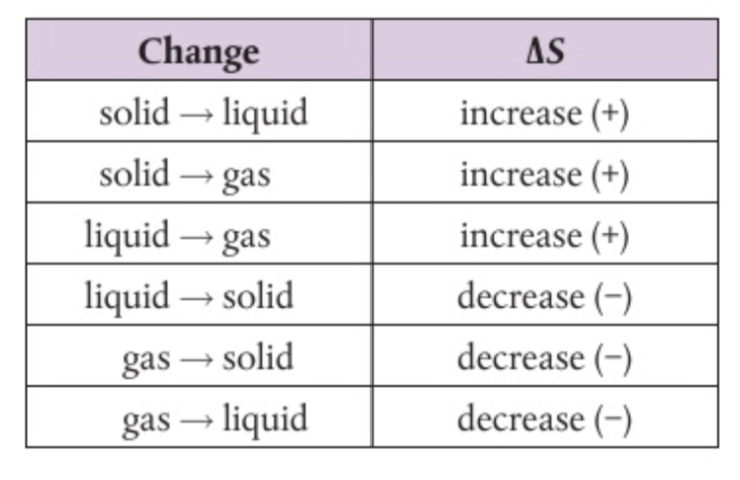
It is the measure of disorderness- a measure of dispersal or distribution of matter and/or energy in a system.
Gas has the highest entropy among all states of matter.
2
New cards
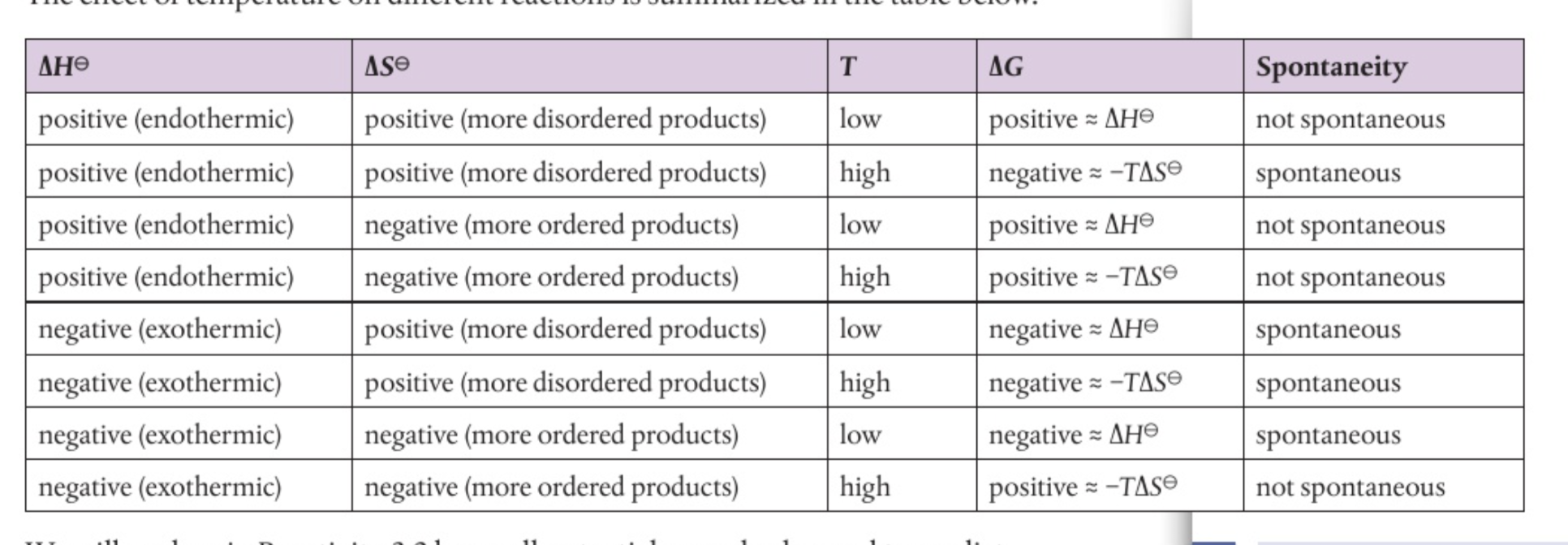
Spontaneous change
Gibb’s energy must be negative
3
New cards
Non-spontaneous change
Gibb’s energy must be positive
4
New cards
Spontaneity
At constant pressure, a change is spontaneous if the change in Gibb’s energy is negative