Chemistry Section 6: Thermochemistry
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Energy
the capacity to do work (w) or to produce heat (q)
Nature of Energy
the concept of “energy” is familiar to us but energy is a bit difficult to define
the energy in the universe is conserved. It can be converted from one form to another, but it can never be created nor destroyed
Potential energy
“stored energy”
It’s the energy associated with the position or composition of an object
Kinetic Energy
“the energy of motion”, that depends on the moving object’s mass adn velocity
KE=1/2mv2
Temperature
a measure of the random motions of the particles in a substance
Heat
involves the transfer of energy between two objects due to a temperature difference
heat (q) flows spontaneously from a hot object to a cooler one
heat does not represent a “substance” contained by an object
The Transfer of Chemical Energy
system = the part of the universe that we’re focusing on (the reaction)
surroundings = everything container/vessel, the lab, etc.
in an exothermic reaction, some of the potential energy stored in the chemical bonds is being converted to thermal energy via heat
so, energy is released to the surroundings
exothermic reactions feel “hot to the touch”
Oppositely, when heat flows into the system, the process is an endothermic reaction; feel cold to the touch because heat is leaving where you are (the surroundings) and going into the reaction (the system)

Thermodynamics
the study of energy and its interconversions
1st law of Thermodynamics
the total energy of the universe is constant
The Internal Energy (E) of a System
the sum of the kinetic and potential energies of all the “particles” in the system (the reaction)
this internal energy (E) can be changed by a flow of work (w), heat (q), or both
E= q+w
the signs (+ or -) of heat (q) and work (w) are identified from the point of view of the system:
if the reaction is endothermic, heat flows into the system and thus heat (q) is (+) q>0
if the reaction is exothermic, heat flows out of the system and thus heat (q) is (-) q<0
if the reaction (system) does work (w) on the surroundings, energy flows out of the system, so work (w) is (-) w<0
if the surroundings do work (w) on the system, energy flows into the system, so work (w) is (+) w>0
Work (w)
the “work” associated with chemical processes is usually work done by gases (through expansion) or work done to gases (through compression)

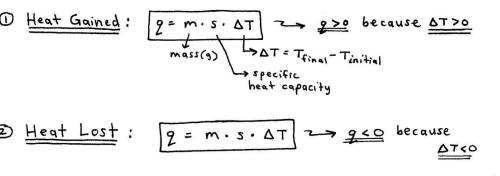
Calorimetry
is based on observing the temperature change when a substance absorbs or gives off heat
some substances require a lot og heat energy to raise their temperature by 1 degree C or 1 k
other substances don’t require very much heat energy to raise their temperature by 1 degree C or 1 K
Heat Capacity ( c )
the amount of energy (as heat) required to raise the temperature of a substance by 1 degree C or 1 k
the higer the “heat energy”, the smaller change in temperature for a given amount of absorbed heat
H2O has a high heat capacity (good coolant)
metals have low heat capacities
if the heat capacity is given per gram of substance, it is called the specific heat capacity (s)
Specific Heat Capacity (s)
the energy (as heat) required to raise the temperature of 1g of a substance by 1 degree C or 1k
the actual measurements of heat gain and/or heat loss are performed using a device called a constant-pressure calorimeter
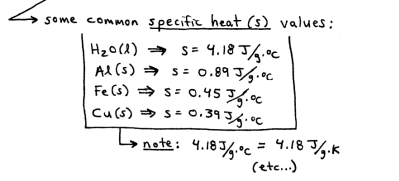
Two important calorimetry equations:
Enthalpy (H)
the energy of a system as heat
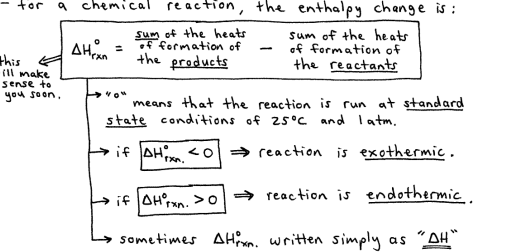
more importantly however, is the change in enthalpy
at constant pressure, the enthalpy change is equal to the energy flow as heat.
in other words, for a reaction at constant pressure, the heat flow (q) is the same as the change in enthalpy
heat of reaction = enthalpy change

Enthalpy: Chemical Reaction
Enthalpy change
is a state function, which means that the H has a certain value irrespective of the methods, steps, or paths in the reaction
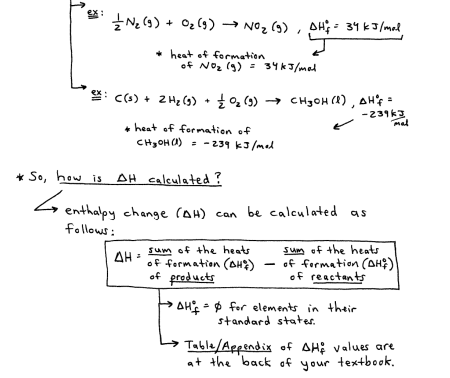
There are many ways to calculate the change in enthalpy for a reaction, two are hess’ law and using standard enthalpies of formation
Hess’ Law
in going from a set of reactanrs to a set of products, the change in enthalpy is the same, regardless of how many steps the process takes
if the reaction is reversed, the sign of enthalpy also gets reversed
if a reaction is multiplied through by a coefficient, then enthalpy also gets multipled through by coefficient
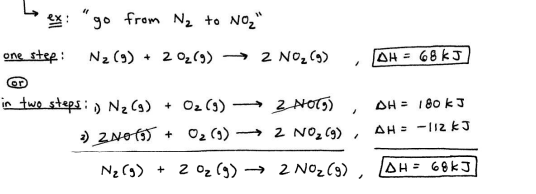
Calculating enthalpy using standard enthalpies of formation
Standard enthalpies of formation = the change in enthalpy that accompanies the formation of 1 mol of a compound from its elements in their standard states