PHY 1.9 Atomic and nuclear phenomena
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
22 Terms
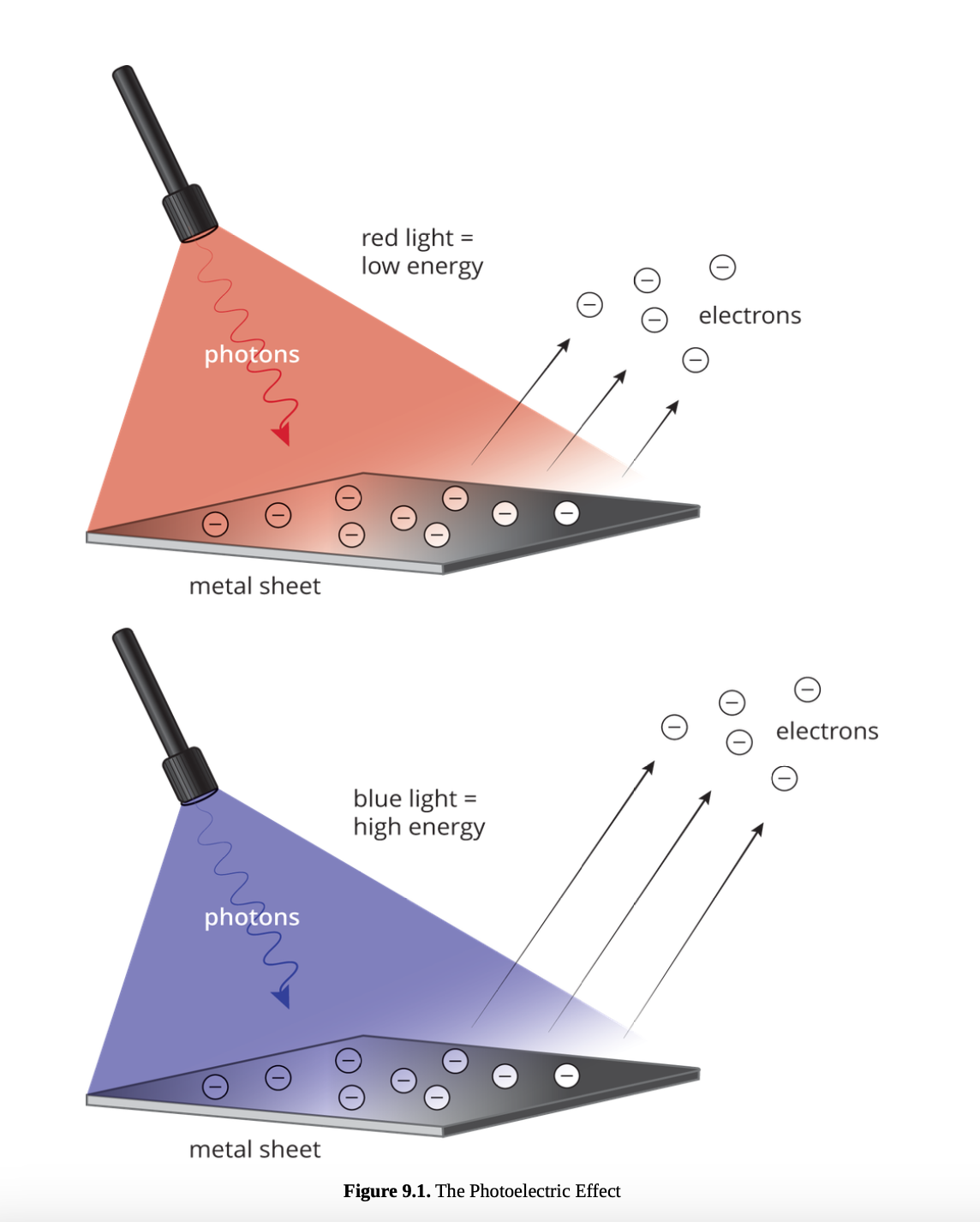
photoelectric effect
is the ejection of an electron from the surface of a metal in response to light.
The ejected electrons create a current; the magnitude of this current is proportional to the intensity of the incident beam of light.
threshold frequency
is the minimum light frequency necessary to eject an electron from a given metal and depends on the type of metal being exposed to the radiation
f the frequency of the incident photon is less than the threshold
frequency (f < fT), then no electron will be ejected because the photons do not have sufficient energy to dislodge the electron from its atom.
But if the frequency of the incident photon is greater than the threshold frequency (f > fT), then an electron will be ejected, and the maximum kinetic energy of the ejected electron will be equal to the difference between hf and hfT (also called the work function).
E = hf
energy of each photon
E is the energy of the photon of light
h is Planck’s constant (6.626 × 10−34 J·s)
f is the frequency of the light.
energy of a photon increases with increasing frequency
c = fλ
waves with higher frequency have shorter wavelengths and higher energy (toward the blue and ultraviolet end of the spectrum);
waves with lower frequency have longer wavelengths and lower energy (toward the red and infrared end of the
spectrum).
Kmax = hf − W
maximum KE ejected electron
Kmax is only achieved when all possible energy from the photon is transferred to the ejected electron.
work function
W = hfT
is the minimum energy necessary to eject an electron from a given metal.
Its value depends on the metal used and can be calculated by multiplying the threshold frequency by Planck’s constant.
The greater the energy of the incident photon above the work function, the more kinetic energy the ejected electron can possess.
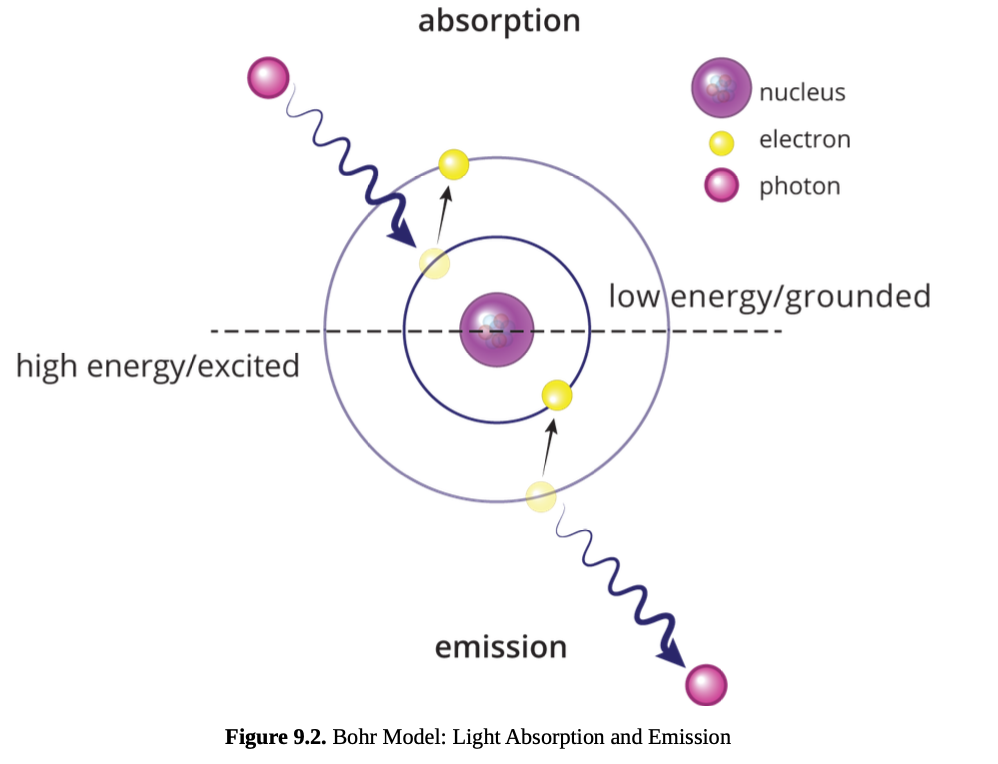
Bohr model of the atom
states that electron energy levels are stable and discrete, corresponding to specific orbits.
An electron can jump from a lower-energy to a higher-energy orbit by absorbing a photon of light of the same frequency as the energy difference between the orbits.
When an electron falls from a higher-energy to a lower-energy orbit, it emits a photon of light of the same frequency as the energy difference between the orbits.
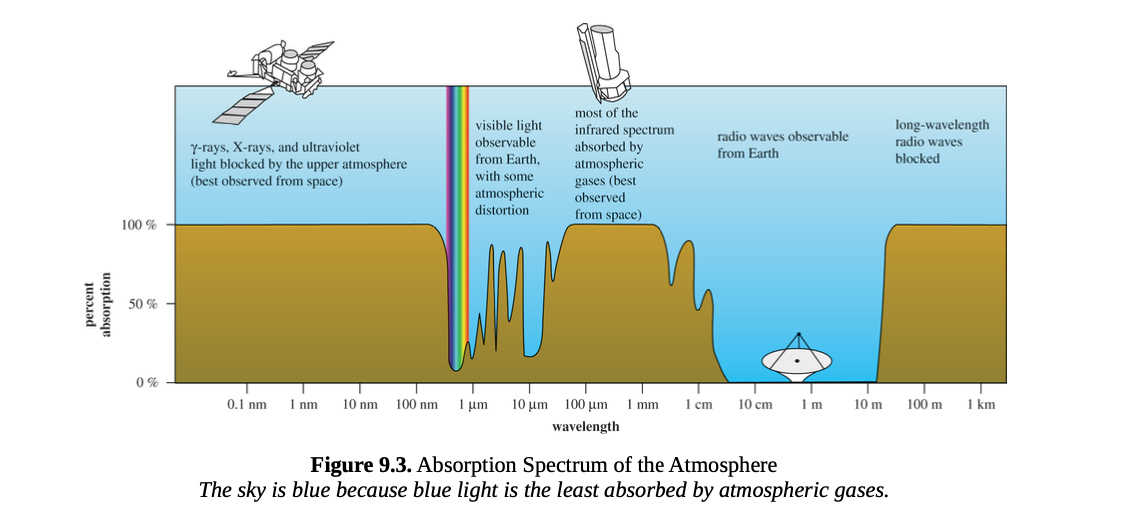
Absorption spectra
may be impacted by small changes in molecular structure
Fluorescence
occurs when a species absorbs high-frequency light and then returns to its ground state in multiple steps. Each step has less energy than the absorbed light and is within the visible range of the electromagnetic spectrum
Nuclear binding energy
is the amount of energy that is released when nucleons (protons and neutrons) bind together.
The more binding energy per nucleon released, the more stable the nucleus.
four fundamental forces of nature
1. + 2. strong and weak nuclear force, which contribute to the
stability of the nucleus
electrostatic forces
gravitation
Although the strong nuclear force is the strongest of the four fundamental forces, it only acts over extremely short distances, less than a few times the diameter of a proton or neutron. The nucleons have to get very close together in order for the strong nuclear force to hold them together.
mass defect
E = mc2
is the difference between the mass of the unbonded nucleons and the mass of the bonded nucleons within the nucleus.
The unbonded constituents have more energy and, therefore, more mass than the bonded constituents.
The mass defect is the amount of mass converted to energy during nuclear fusion.
fusion
occurs when small nuclei combine into larger nuclei.
fission
occurs when a large nucleus splits into smaller nuclei.
Energy is released in both fusion and fission because the nuclei formed in both processes are more stable than the starting nuclei.
radioactive decay
decay is the loss of small particles from the nucleus.
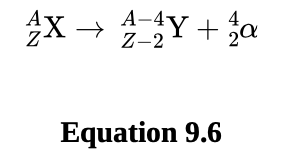
Alpha (α) decay
is the emission of an alpha particle 4/2 He which is a helium nucleus.
Alpha particles do not have any electrons, so they carry a charge of +2.
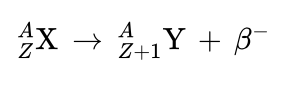
beta negative decay (β−)
is the decay of a neutron into a proton, with emission of an electron (e− , β−) and an antineutrino (ν).
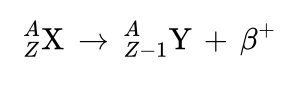
Beta-positive (β+) decay
also called positron emission, is the decay of a proton into a neutron, with emission of a positron (e+, β+) and a neutrino (ν).
Gamma (γ) decay
is the emission of a gamma ray, which converts a high-energy nucleus into a more stable nucleus.
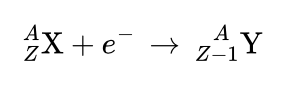
electron capture
is the absorption of an electron from the inner shell that combines with a proton in the nucleus to form a neutron.
half life
is the amount of time required for half of a sample of radioactive nuclei to decay
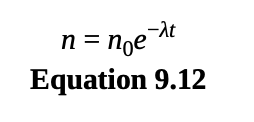
exponential decay
the rate at which radioactive nuclei decay is proportional to the number of nuclei that remain
where n0 is the number of undecayed nuclei at time t = 0.