Pre-AP Chem Molecular Geometry
1/27
Earn XP
Description and Tags
includes electron and molecular geometry names. 2024 fall final Pre-AP Chem
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms

What is the electron and molecular geometry of a molecule with 2 electron dense areas and no lone pairs?
Linear Linear
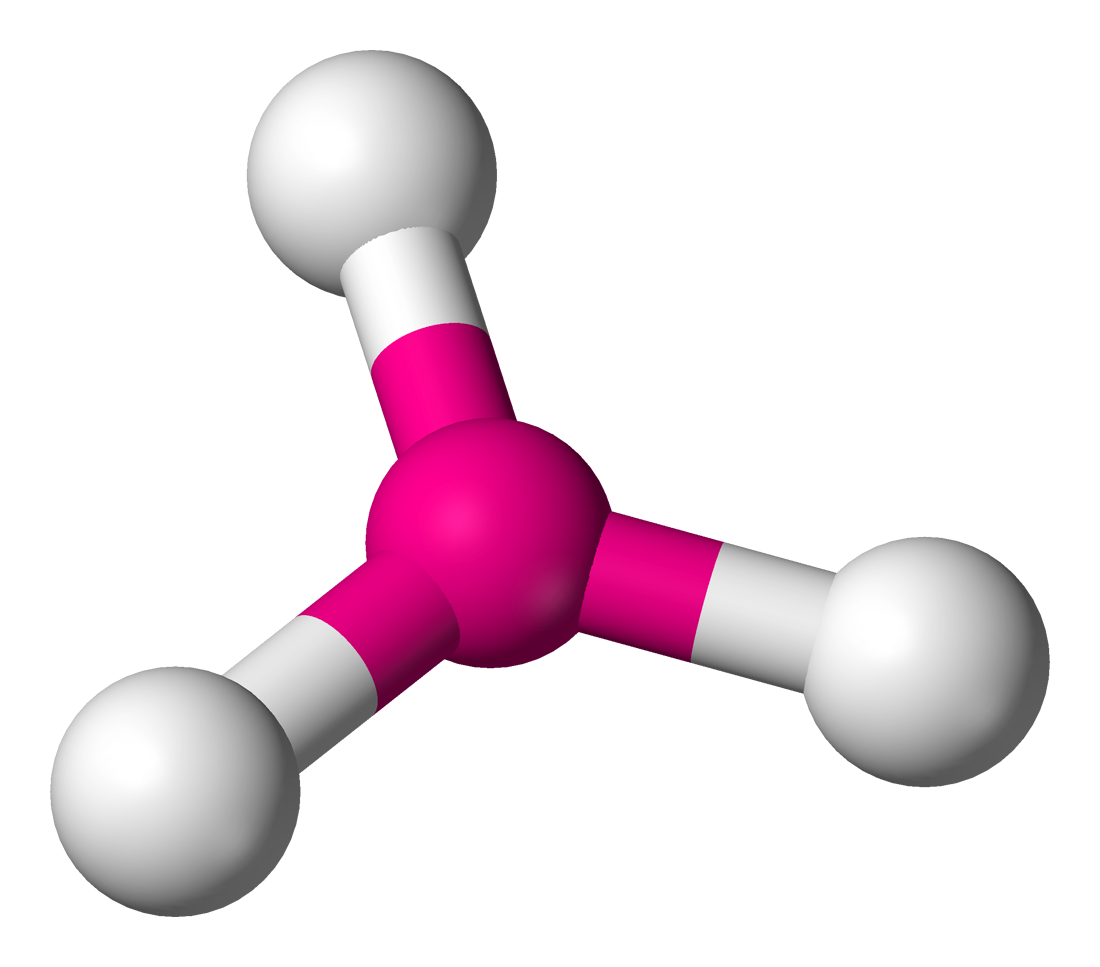
What is the electron and molecular geometry of a molecule with 3 electron dense areas and no lone pairs?
Trigonal Planar Trigonal Planar
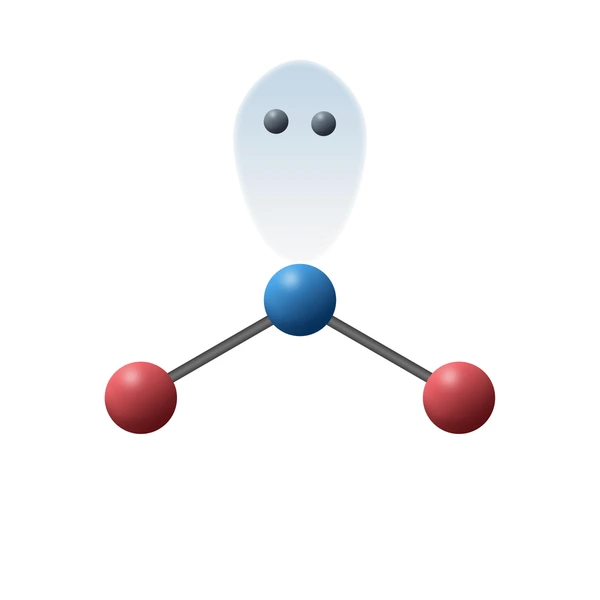
What is the electron and molecular geometry of a molecule with 3 electron dense areas and 1 lone pair?
Trigonal Planar Bent
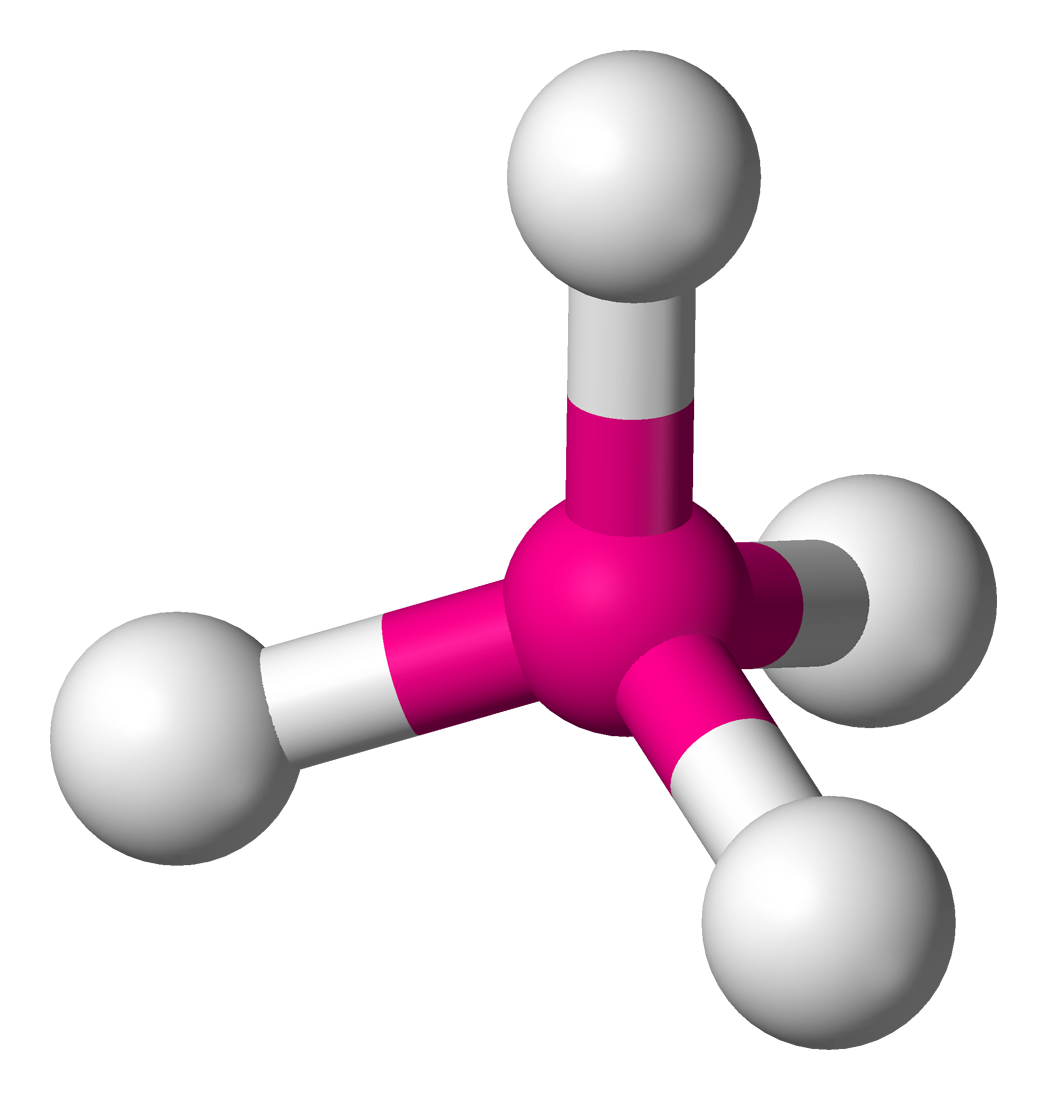
What is the electron and molecular geometry of a molecule with 4 electron dense areas and no lone pairs?
Tetrahedral Tetrahedral

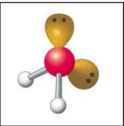
What is the electron and molecular geometry of a molecule with 4 electron dense areas and 1 lone pair?
Tetrahedral Trigonal Pyramidal
What is the electron and molecular geometry of a molecule with 4 electron dense areas and 2 lone pairs?
Tetrahedral Bent
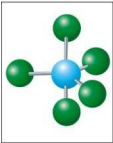
What is the electron and molecular geometry of a molecule with 5 electron dense areas and no lone pairs?
Trigonal Bipyramidal Trigonal Bipyramidal
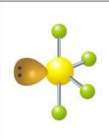
What is the electron and molecular geometry of a molecule with 5 electron dense areas and 1 lone pair?
Trigonal Bipyramidal Seesaw/Sawhorse
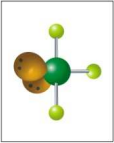
What is the electron and molecular geometry for a molecule with 5 electron dense areas and 2 lone pairs?
Trigonal Bipyramidal T-Shaped
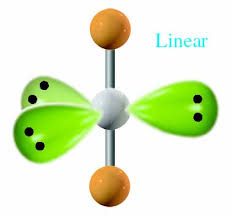
What is the electron and molecular geometry of a molecule with 5 electron dense areas and 3 lone pairs?
Trigonal Bipyramidal Linear
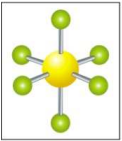
What is the electron and molecular geometry for a molecule with 6 electron dense areas and no lone pairs?
Octahedral Octahedral
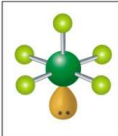
What is the electron and molecular geometry for a molecule with 6 electron dense areas and 1 lone pair?
Octahedral Square Pyramidal
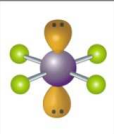
What is the electron and molecular geometry of a molecule with 6 electron dense areas and 2 lone pairs?
Octahedral Square Planar
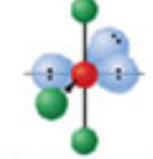
What is the electron and molecular geometry of a molecule with 6 electron dense areas and 3 lone pairs
Octahedral T-shaped
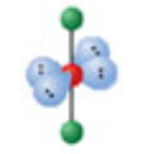
What is the electron and molecular geometry of a molecule with 6 electron dense areas and 4 lone pairs?
Octahedral Linear
What is the bond angle of a linear linear molecule?
180
What is the bond angle of a trigonal planar molecule?
120
What is the bond angle of a trigonal planar bent molecule?
118
what is the bond angle of a tetrahedral tetrahedral molecule?
109.5
What is the bond angle of a tetrahedral trigonal pyramidal molecule?
107
what is the bond angle of a tetrahedral bent molecule?
105
What are the bond angles of a trigonal bipyramidal molecule?
90, 120, 180
What is the bond angle of an octahedral octahedral molecule?
90
What is the bond angle of an octahedral square planar molecule?
90
Expanded Octets: how many electrons is Beryllium satisfied with?
4
Expanded Octets: how many electrons is Boron satisfied with?
6
Expanded Octets: how many electrons does Sulfur expand to?
12
Expanded Octets: How many electrons does Phosphorus usually expand to?
10