Biology Midterm 1 (Ch 1-Ch 6)
1/145
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
146 Terms
What are the characteristics of living organisms?
1. The have cells
2. Information use
3. Evolution
4. Replication
5. Energy
Describe the characteristic of life: Cells
Membrane bound unit in an aqueous solution containing fundamental molecules
Describe the characteristic of life: Replication
An organism is able to reproduce, have offsping for other generations
Describe the characteristic of life: Information usage
Has hereditary material (DNA) that is able to be processed with traits being expressed
Describe the characteristic of life: Energy
All living things require energy (ATP, glucose, etc.) in order to survive.
Describe the characteristic of life: Evolution
An organism can change and adapt to an enviornment, each generation expressing more of the favorable trait allowing them to survive.
Cell Theory
All organisms are made of cells, those cells come from preexisting cells (no spontanous generation)
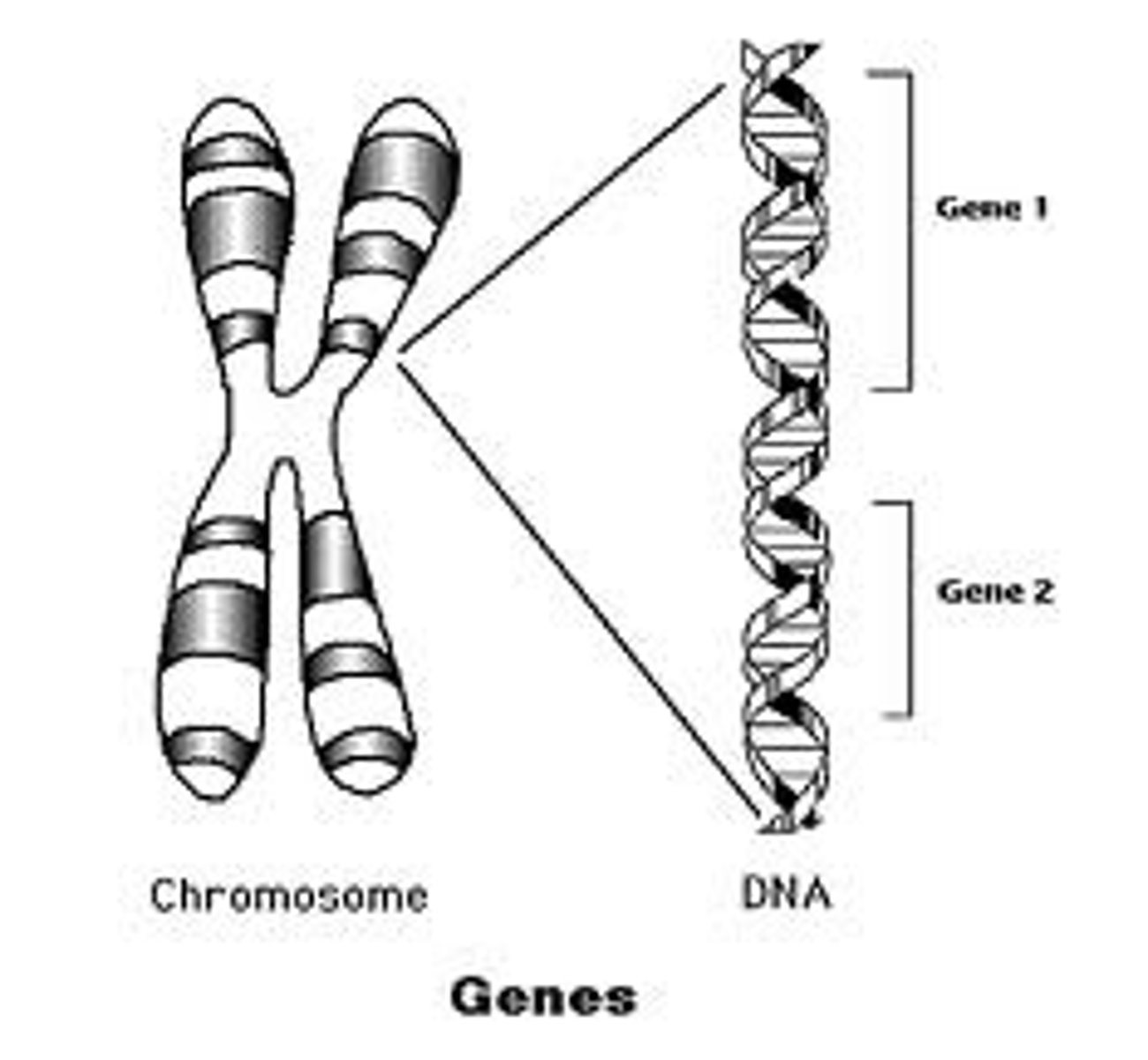
Chromosome Theory of Inheritence
Heredity is encoded in genes --> genes are units on a chromosome --> a chromosome is DNA.
As such, DNA is hereditary material in which genes are segmented DNA that "codes" a cell product.
How does evolution occur via natural selection?
The traits that allow a species to survive and procreate will be passed down and those that receive the favorable traits will be allowed to survive.
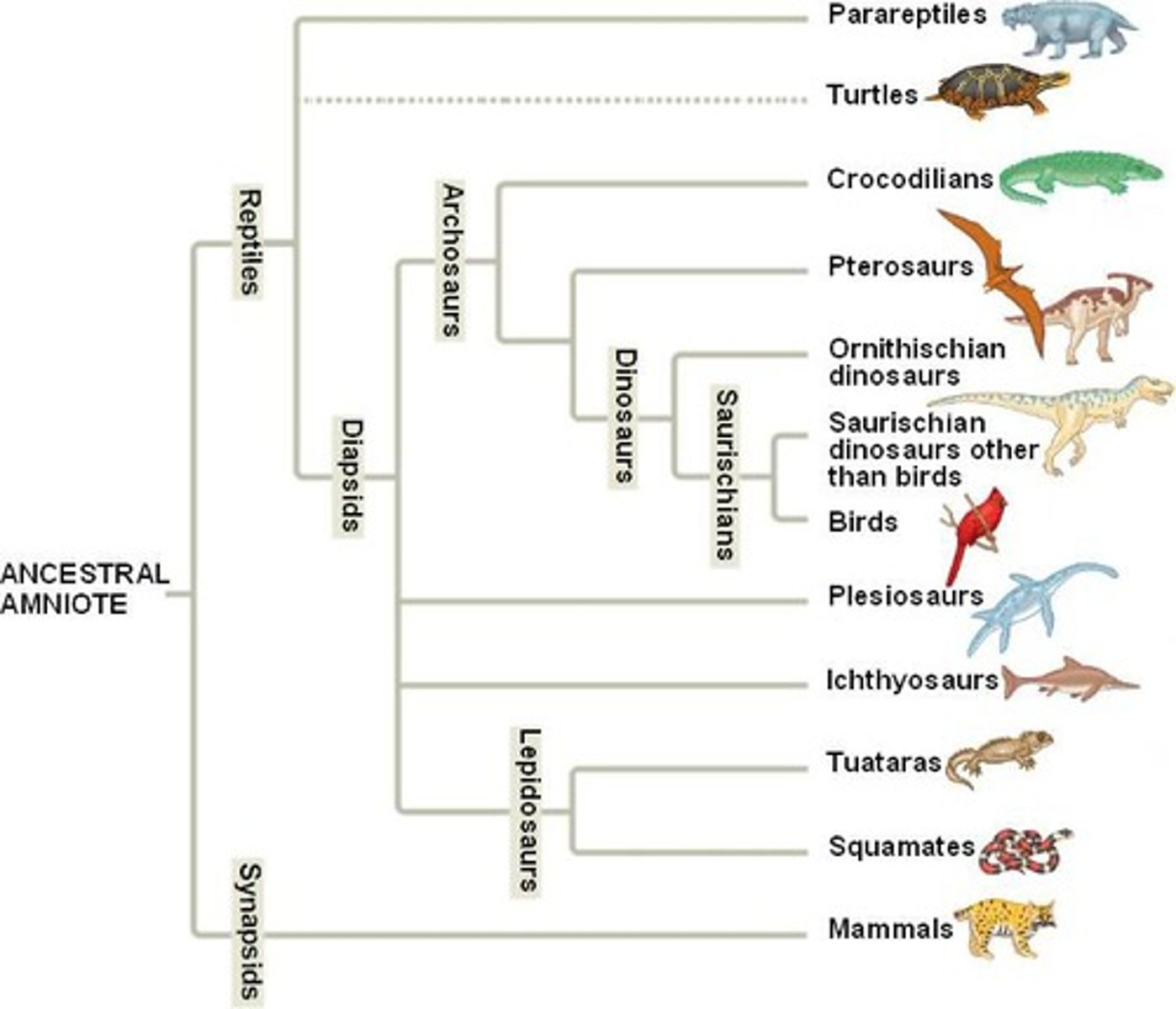
How would you determine relatedness on a phylogenic tree?
You look at the tree, closer the common ancestor (branch) means the more closely related the two species are.
Which animal is more closely related to birds? Plesiosaurs or Archosaurs?
(Flip card over to review image)
Archosaurs
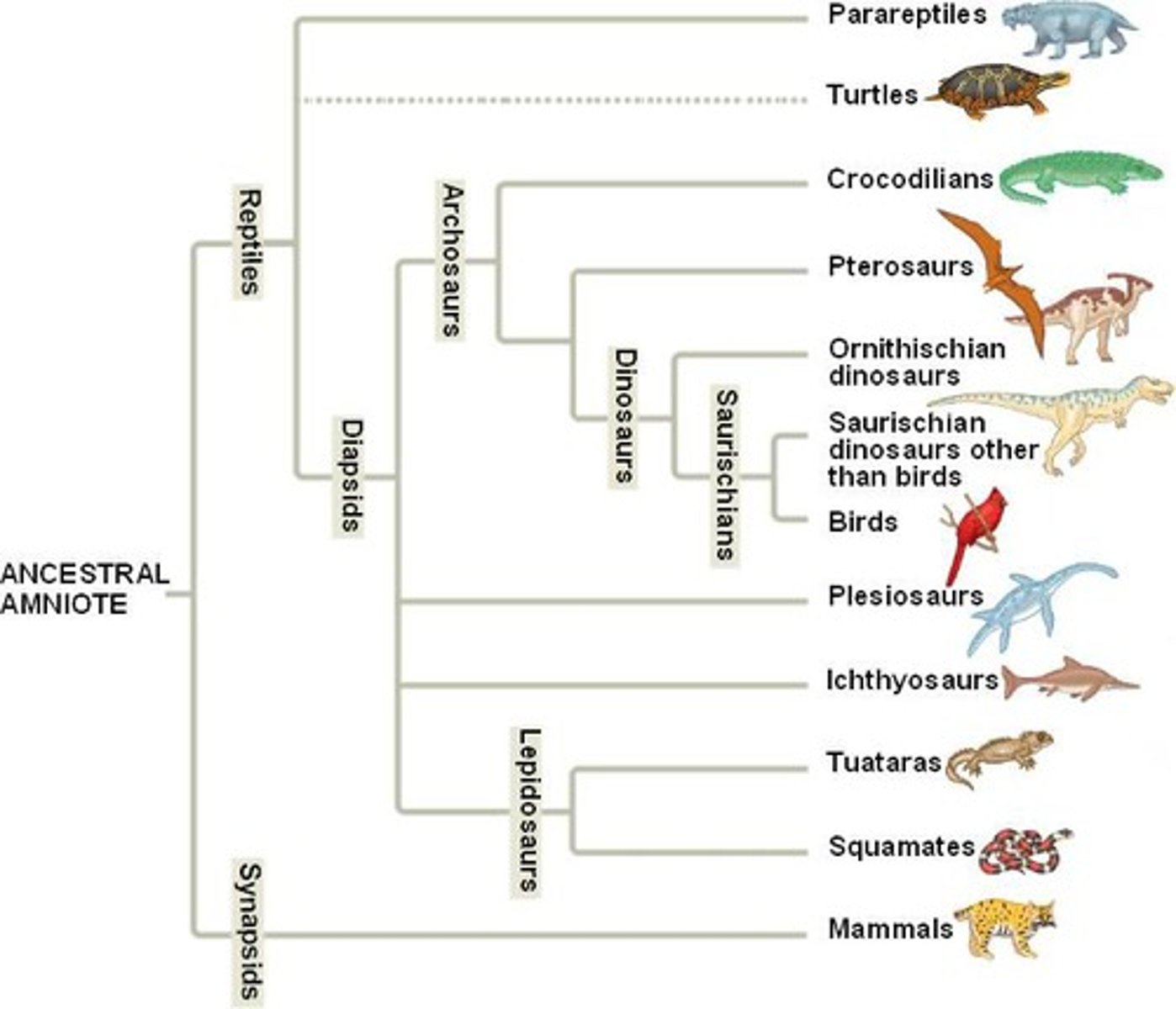
Which animal is more closely related to crocodilians? Parareptiles or Petrosaurs?
(Flip card over to review image)
Petrosaurs
What is the process of science
1. Question
2. Hypothesis
3. Predictions
4. Design experiment
5. Collect data
6. Analyze results
7. Conclusions
Describe the process of science: Question
The initial inquiry or problem that a scientist wants to answer through experimentation
Describe the process of science: Hypothesis
A testable statement that explains a phenomenon or a set of observations.
Describe the process of science: Prediction
A measurable or observable result of an experiment based on a particular hypothesis. A correct prediction provides support for the hypothesis being tested.
Describe the process of science: Design Experiment
A powerful scientific tool in which researchers test the effect of a single, well-defined factor on a particular phenomenon.
Describe the process of science: Collect Data
Where a researcher actively gathers information or measurements related to their research question, usually through observation, experimentation, or surveys, to test their hypothesis and ultimately draw conclusions based on the collected evidence
Describe the process of science: Analyze Results
The process of interpreting data to find patterns, trends, and other relationships. Often relating back to the hypothesis.
Describe the process of science: Conclusions
A statement that summarizes the results of an experiment and is based on observations and measurements.
What are the components of good experimental design?
1. Manipulation
2. Control
3. Random assignment
4. Random selection
Describe the component of experimental design: Manipulation
Purposeful change done to an environment
Describe the component of experimental design: Control
Aomething to compare normal results to
Describe the component of experimental design: Random Assignment
Strengthens validity and avoids bias by ensuring it's not a difference in population type
Describe the component of experimental design: Random Selection
Eliminates bias ensuring a representative sample. This way data can be generalized
What is the Central Dogma of Biology
It shows how genetic information flows out of a cell body.
DNA --> RNA --> Protein
What are the 3 domains of life?
1. Bacteria (Prokaryotes; don't contain a nucleus)
2. Archaea (Prokaryotes; don't contain a nucleus)
3. Eukarya (Eukaryotes; contain a nucleus)
How do you know the number of protons in an atom?
By atomic number
How do you know the number of neutrons in an atom?
atomic mass - atomic number (number of protons)
How do you know the number of electrons in an atom?
Same as the number of protons
**ONLY in a neutrally charged atom
Why do different isotopes of the same element vary in atomic mass?
They have a different amount of neutrons to make the weight change.
(# of neutrons can still be found by atomic mass - atomic number)
Describe a polar-covalent bond
Between 2 non-metals, determined by electronegativity, electrons not shared equally (ex: molecules)
**More electronegative, the more electrons they want.
**Partially charged
Describe a nonpolar-covalent bond
2 non-metals with no charge (cation & anion), electrons shared equally (ex: molecules)
Describe an ionic bond
Between metal and non-metal (based on charge), electrons transferred from 1 atom to another to gain a full shell. (cation and anion), attracted to opposite charges.
**Fully charged
Describe a chemical bond
an atom gets full outer shell, attractions bind atoms together (ex: compounds)
Describe a hydrogen bond
a weak attraction between a hydrogen atom with a partialpositive charge and another atom with a partial negativecharge (usually O or N)
Which type of bond is the strongest?
Covalent bonds are the strongest
Memory aid: they share themselves like a partner
What types of bonds can form hydrogen bonds
polar-covalent bonds
What are the most electronegative elements?
F, O, N, Cl.
**If you see any of those in an r-group then you know it's polar
What precentage of cells is made of water?
75% of cells are water
**All of the chemical reactions in the body take place inan aqueous (water-based) environment
What is the relationship of polar molecules to water?
Polar molecules dissolve in water (hydrophilic)
**This is because they have some charge and want to hydrogen bond with water molecules
What is the relationship of nonpolar molecules to water?
Nonpolar molecules DON'T dissolve in water (hydrophobic)
**This is because they don't have charge and want to be left in stable equilibrium
Do ionic bonds dissolve in water?
Yes! They dissolve by separating into ions and interacting with the partial charges of water
List the properties of water
1. Universal solvent
2. Like dissolves like (acts as a solvent in solution)
3. Cohesion
4. Adhesion
5. Able to absorb large amounts of energy
6. Denser as a liquid than a solid
Why is water the universal solvent?
It dissolves more substances than any other liquid.
Participates in hydrogen bonding
Describe the water property: Cohesion
Attraction between water molecules leads to high surface tension
**This leads to high surface tension
Describe the water property: Adhesion
Attraction between water and other molecules
**Sticks to other surfaces well
Why does ice float on water?
Ice is less dense than water. H2O in it's liquid form is as dense as the molecule can get
Why can water absorb large amounts of energy?
1. Has a high specific heat value (Amount of energy required to raise the temperature of 1 gram by 1 C)
2. Has a high heat of vaporization (Amount of energy required to change 1 gram from a liquid to a gas (evaporate))
What happens during a chemical reaction to make an acid?
It gives up a proton (H+) during a chemical reaction
What happens during a chemical reaction to make a base?
acquires a proton (H+) during a chemical reaction, or gives up a hydroxide (OH-)
What is the charge of a base?
Bases are negative because they want to attract (gain) H+ protons in reactions
What is the charge of an acid?
Bases are positive because they want to lose (give up) H+ protons in reactions
How is pH determined?
pH is determined by the concentration on protons (H+)
What is the relationship between pH and H+
Inverse relationship
pH goes down as H+ goes up
What is potential energy?
stored energy
What is potential chemical energy?
potential energy stored in bonds
**more weak and unstable bonds in a molecule means more chemical potential energy
What is kenetic energy?
energy of motion
What is thermal energy?
kinetic energy associated with the random movement of atoms or molecules
What is entropy?
Amount of disorder/randomness; how much the energy within is dispersed
**More molecules in one space=more disorder
What is an organic molecule?
molecule that contains carbon bonded to other elements, linked in a chain or ring
Name the functional groups of organic compounds
1. Amino
2. Carboxyl
3. Carbonyl
4. Hydroxyl
5. Phosphate
6. Sulfhydryl
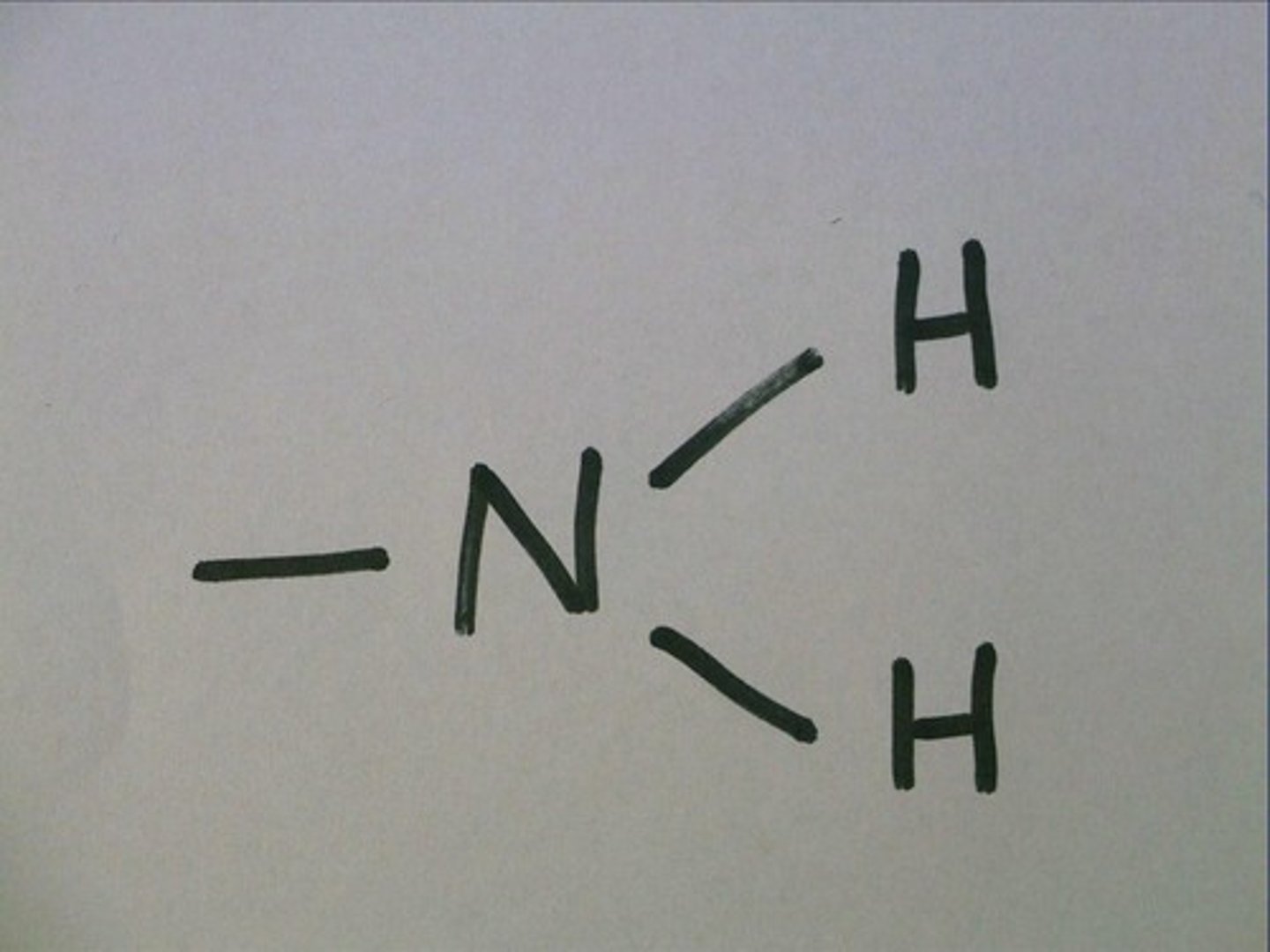
amino group
A functional group that consists of a nitrogen atom bonded to two hydrogen atoms
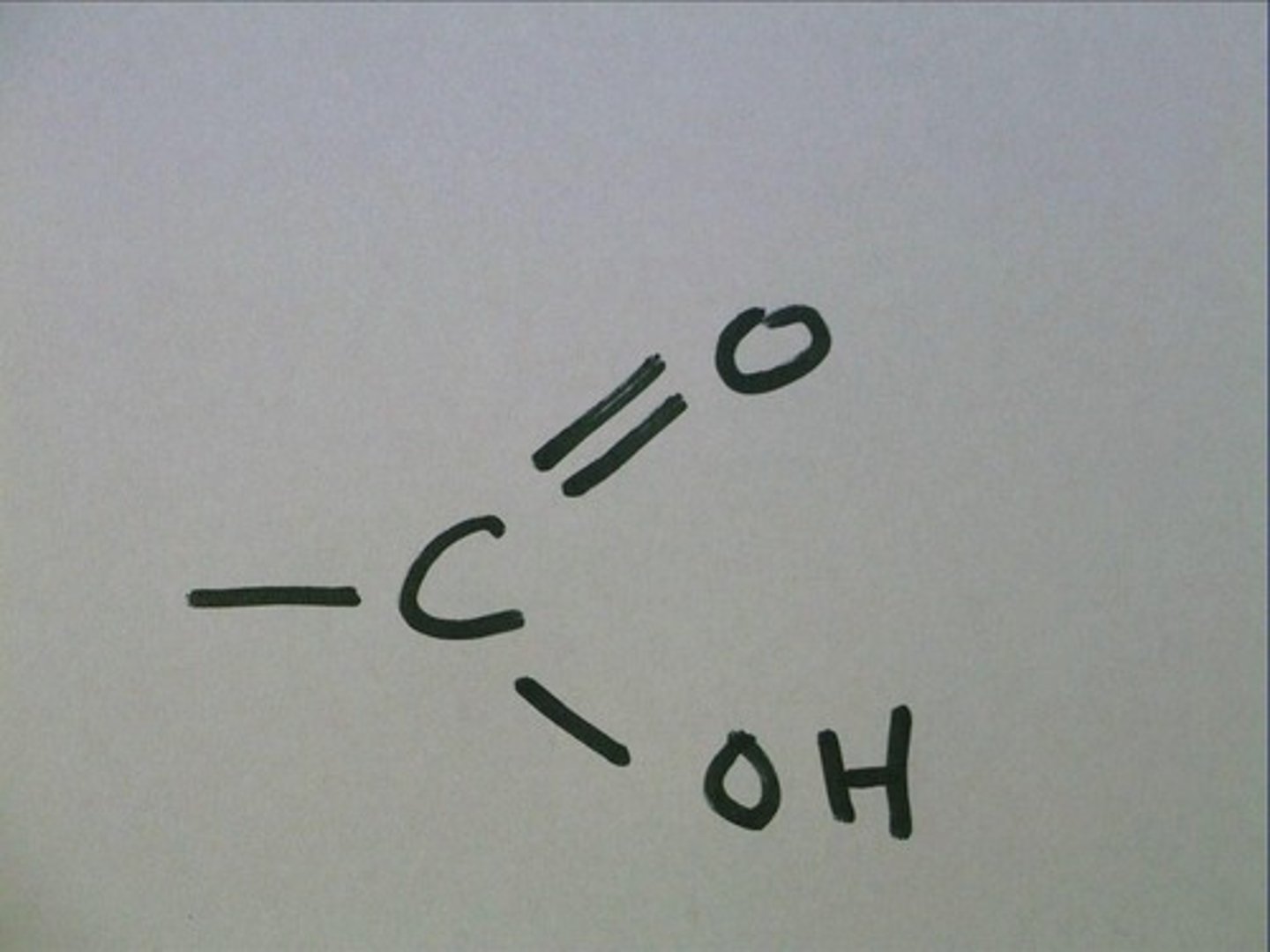
carboxyl group
A functional group present in organic acids and consisting of a single carbon atom double-bonded to an oxygen atom and also bonded to a hydroxyl group.
carbonyl group
a functional group consisting of a carbon atom linked by a double bond to an oxygen atom
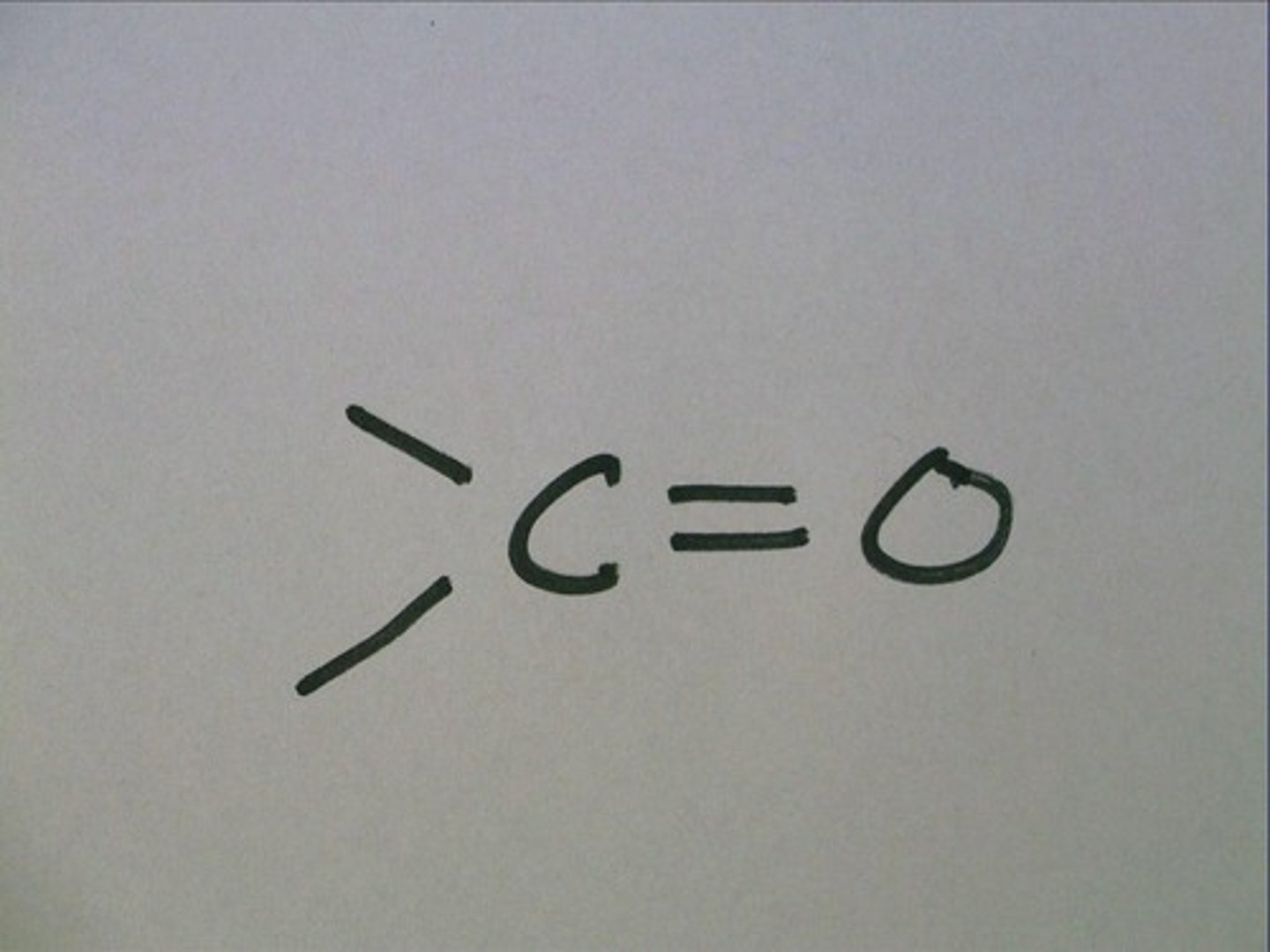
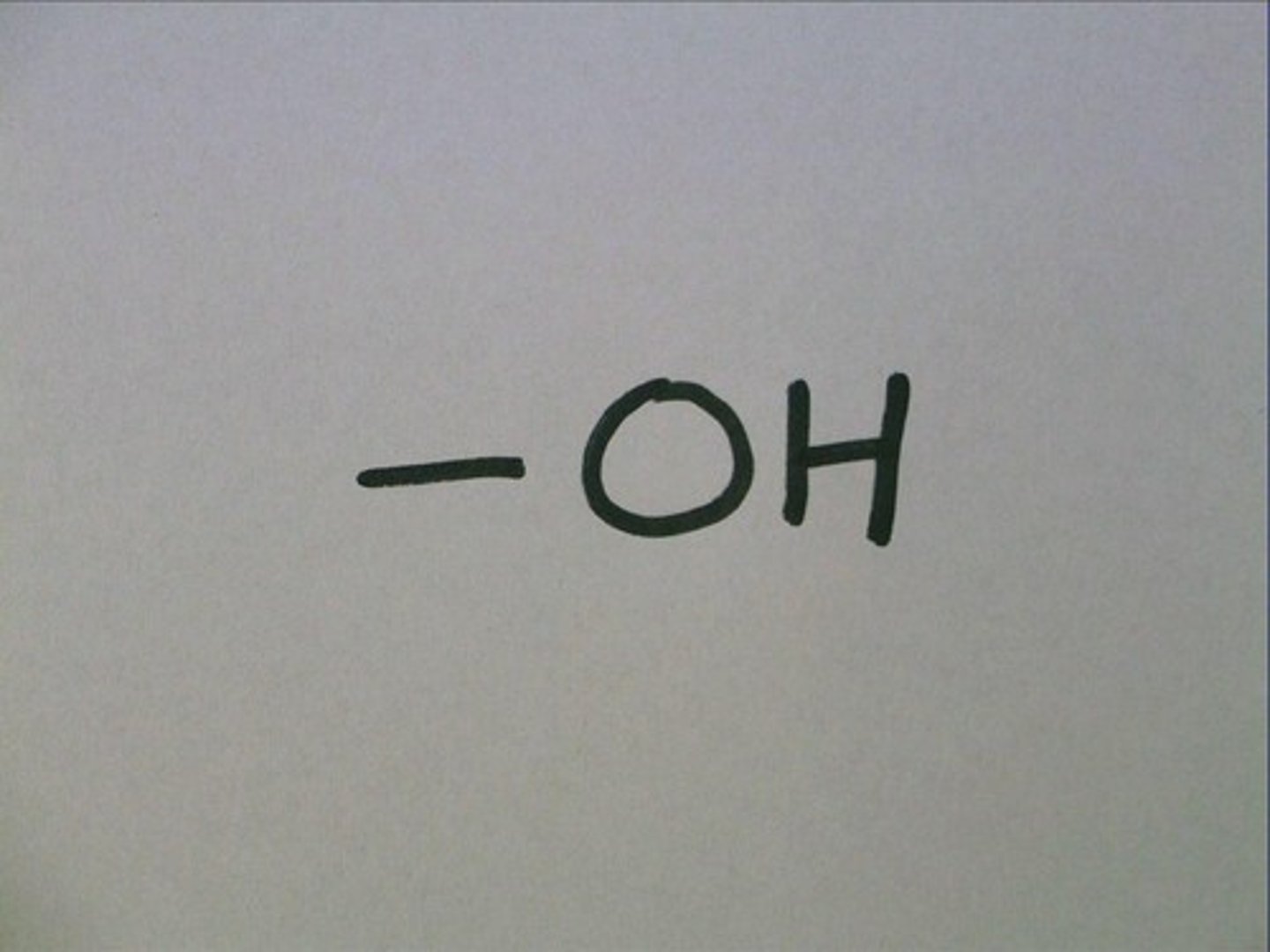
hydroxyl group
A fucnctional group consisting of an oxygen atom bonded to a hydrogen atom.
phosphate group
A functional group consisting of a phosphorus atom covalently bonded to four oxygen atoms
**Often has a ton of energy
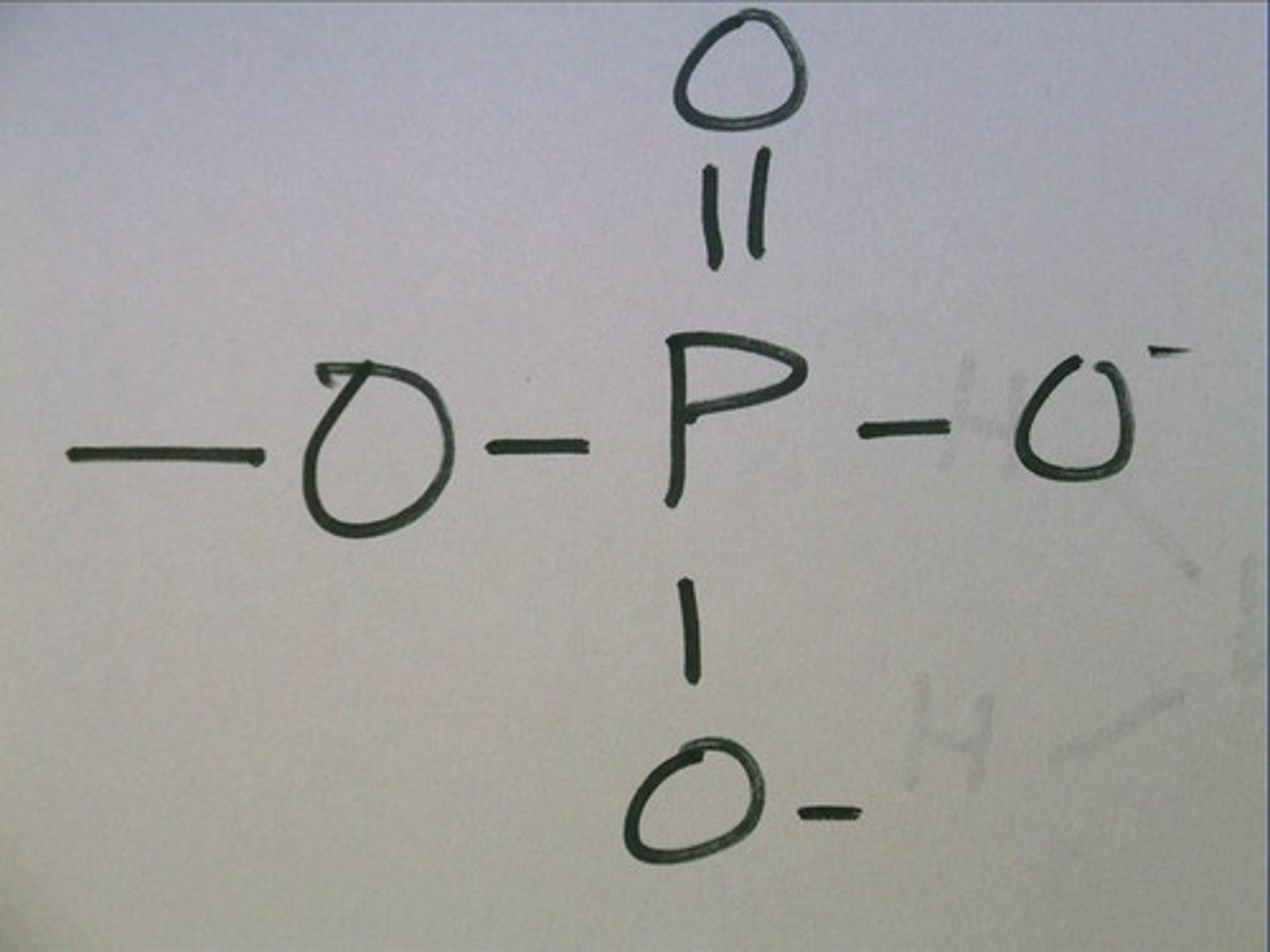
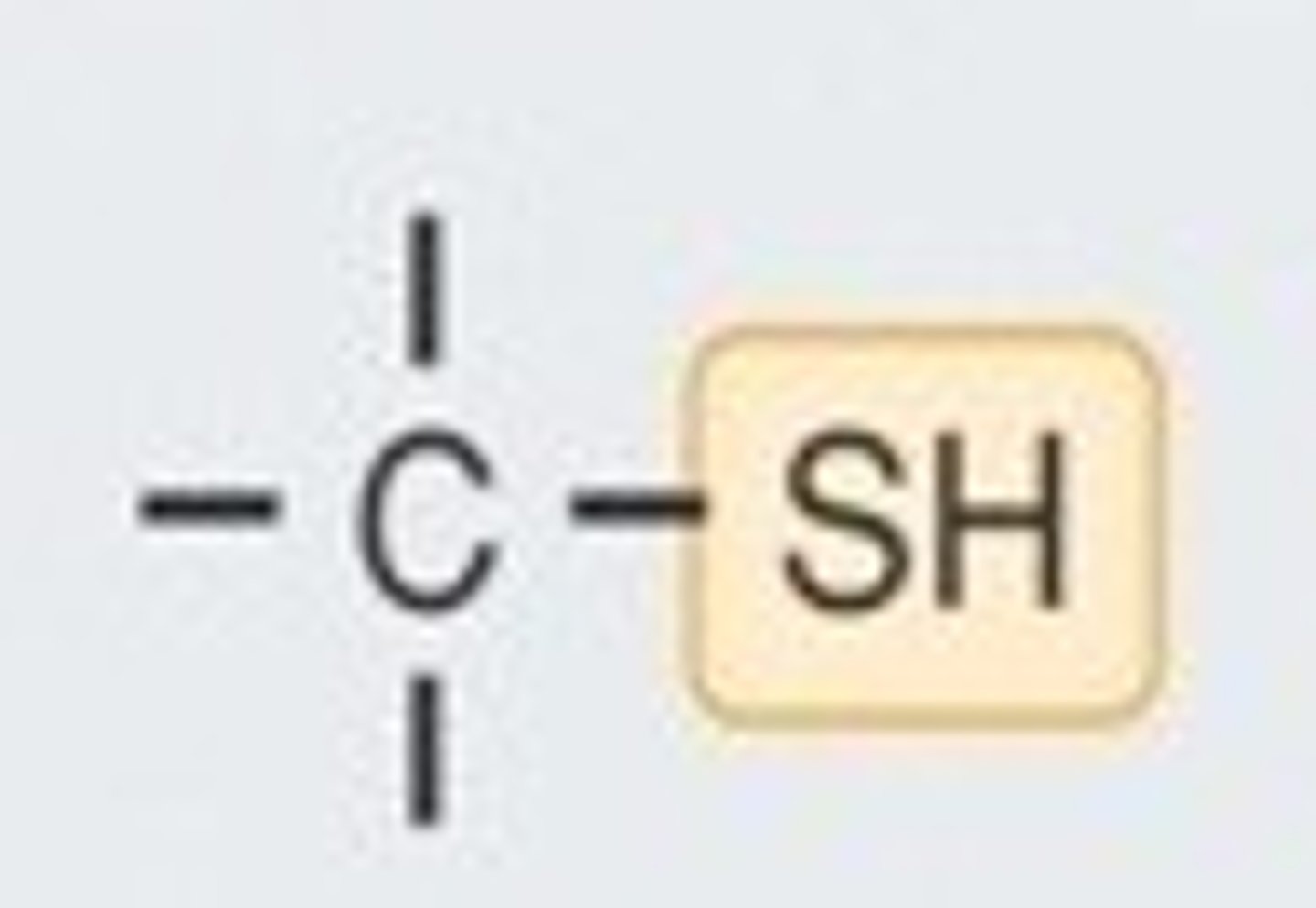
sulfhydryl group
A functional group consisting of a sulfur atom bonded to a hydrogen atom (—SH).
What are the macromolecules of biology?
4 classes
1. Proteins
2. Nucleic Acids
3. Carbohydrates
4. Lipids
Which macromolecules are polymers?
All of them except lipids, so carbohydrates, proteins and nucleic acids.
How are polymers formed?
Polymerization happens through condensation (dehydration).
New bonds result in a loss of water. This takes energy to do because you have to break bonds to form a new compound.
How are polymers broken?
Hydrolysis. You add water to break the bonds present.
What are the basics of proteins
Polymer: polypeptide
Monomer: amino acid
Linked by: peptide bond
Functions: Everything except energy storage!
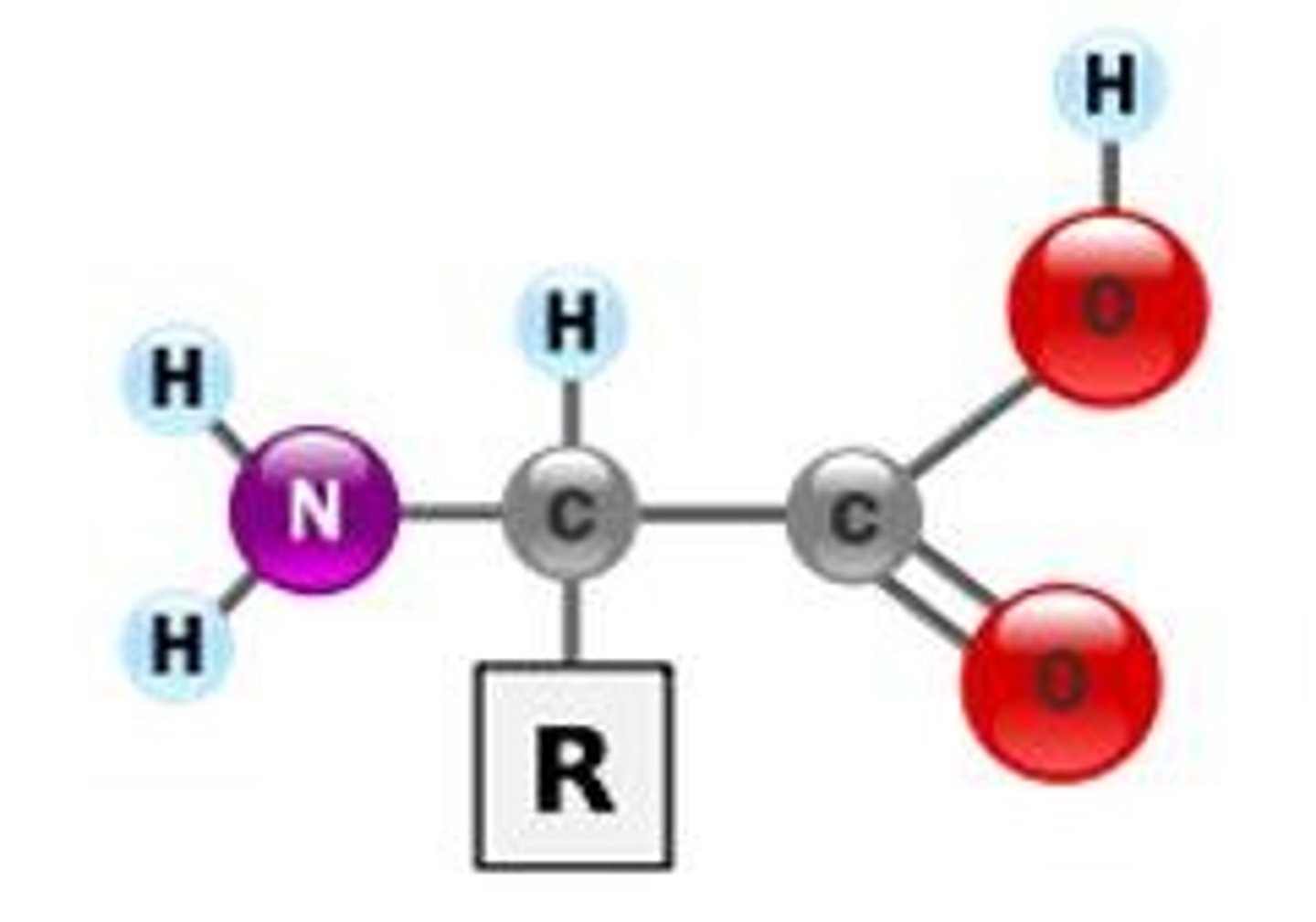
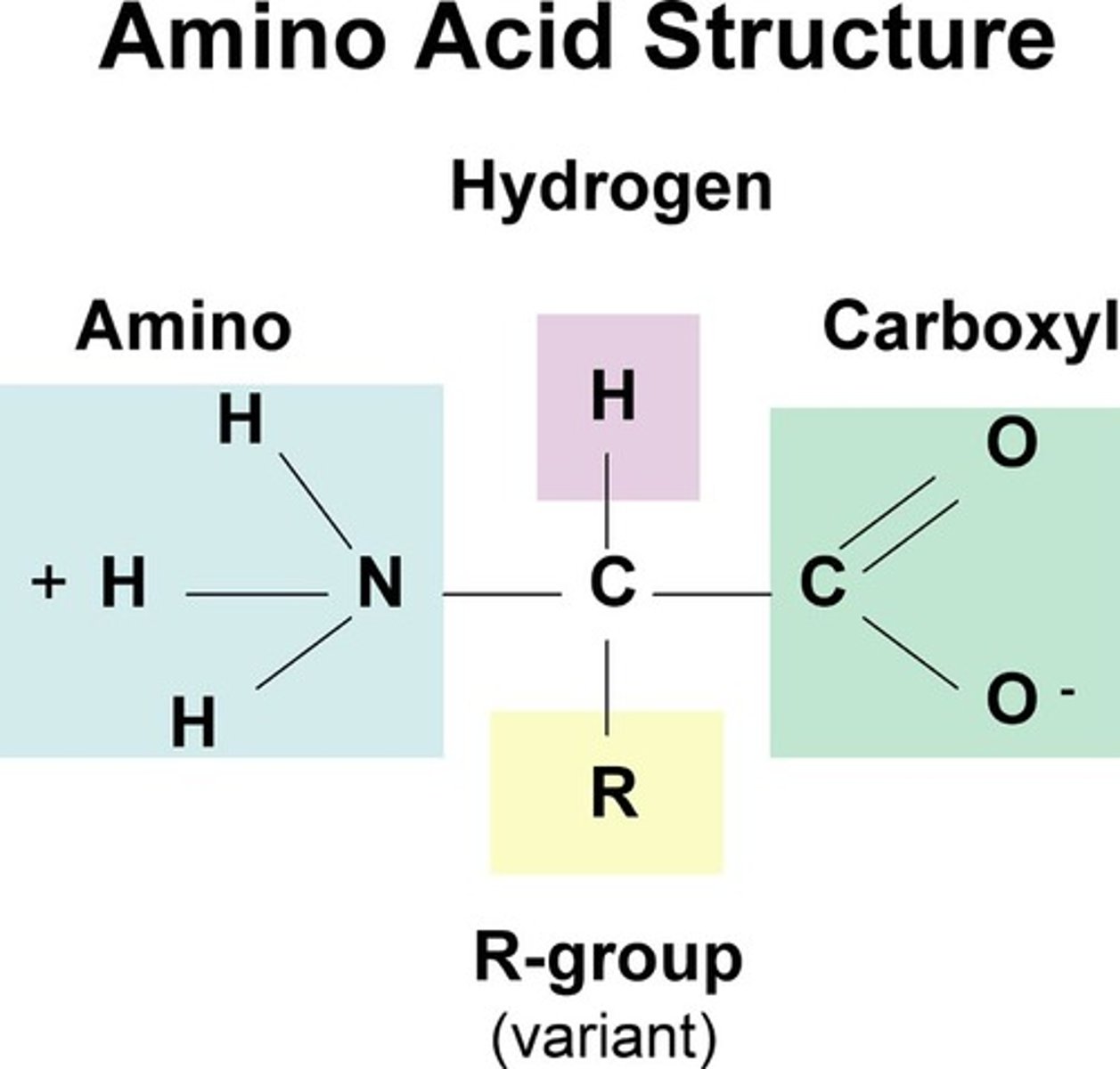
What is the structure of an amino acid?
Amino group (N-terminus), Carboxyl group (right side), Side chain (R-group), all connected to a central carbon
How do you know an amino acid is acidic?
The R-group will have a charge, the charge will be negative
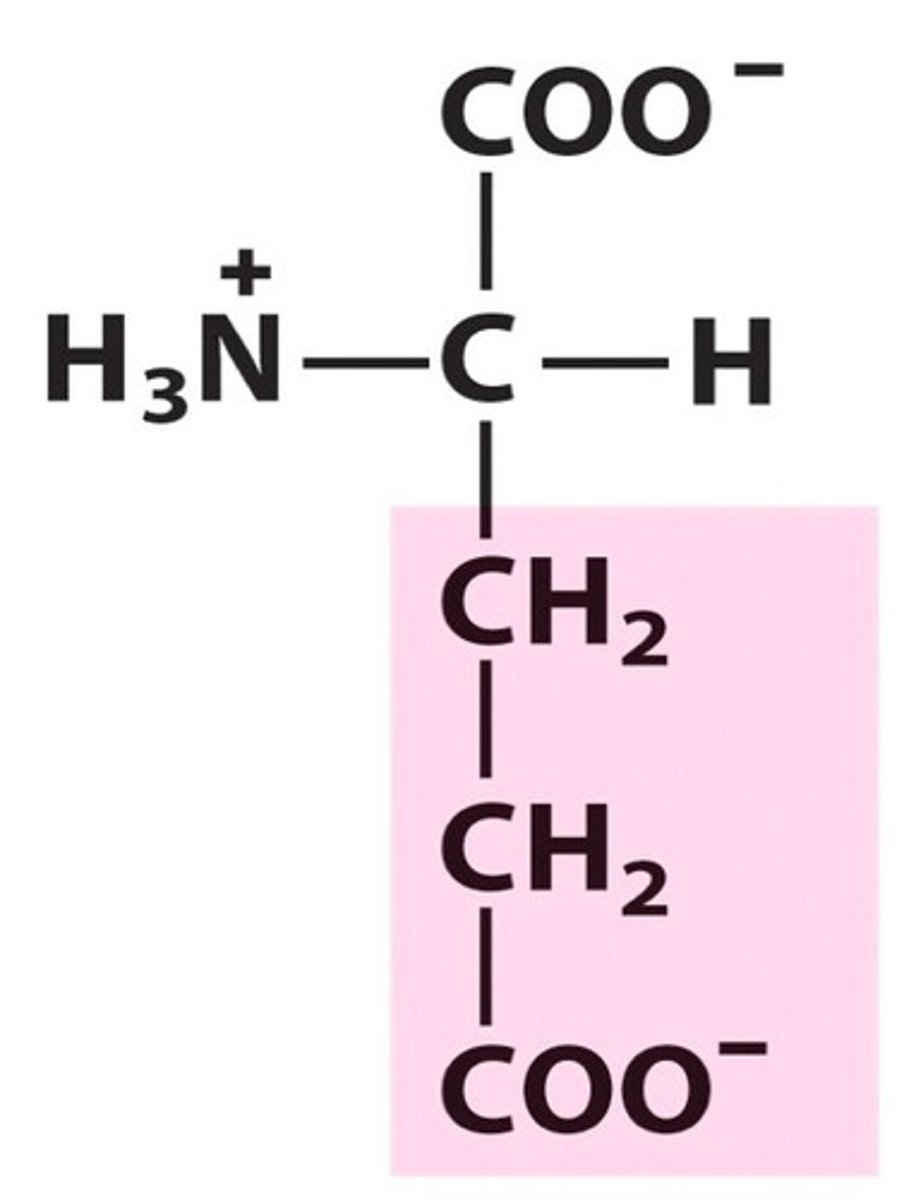
How do you know an amino acid is basic?
The R-group will have a charge, the charge will be positive
**memory aid: it's positive to be a basic bitch
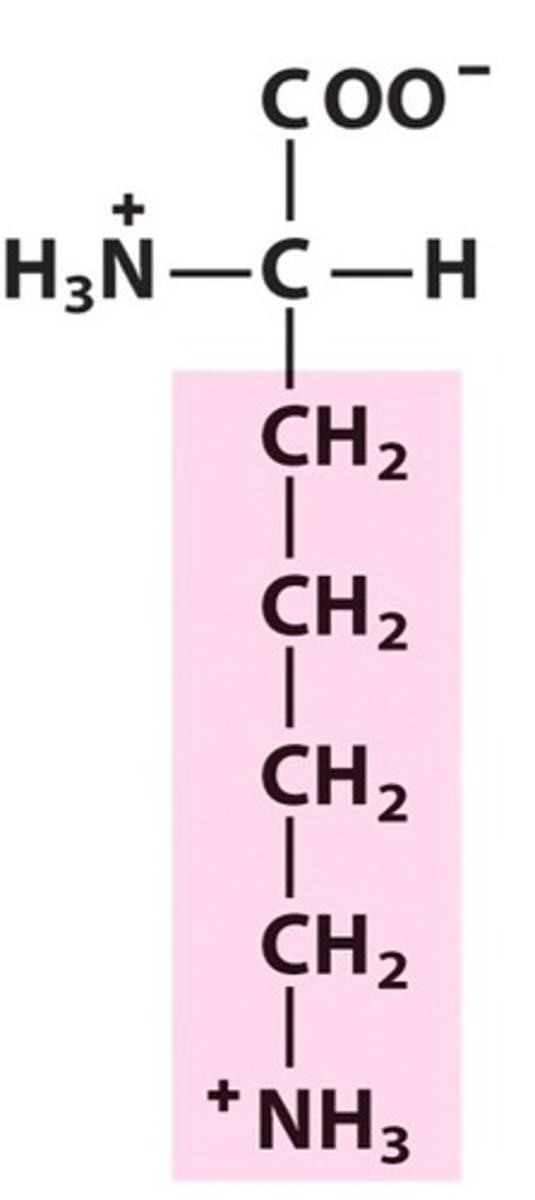
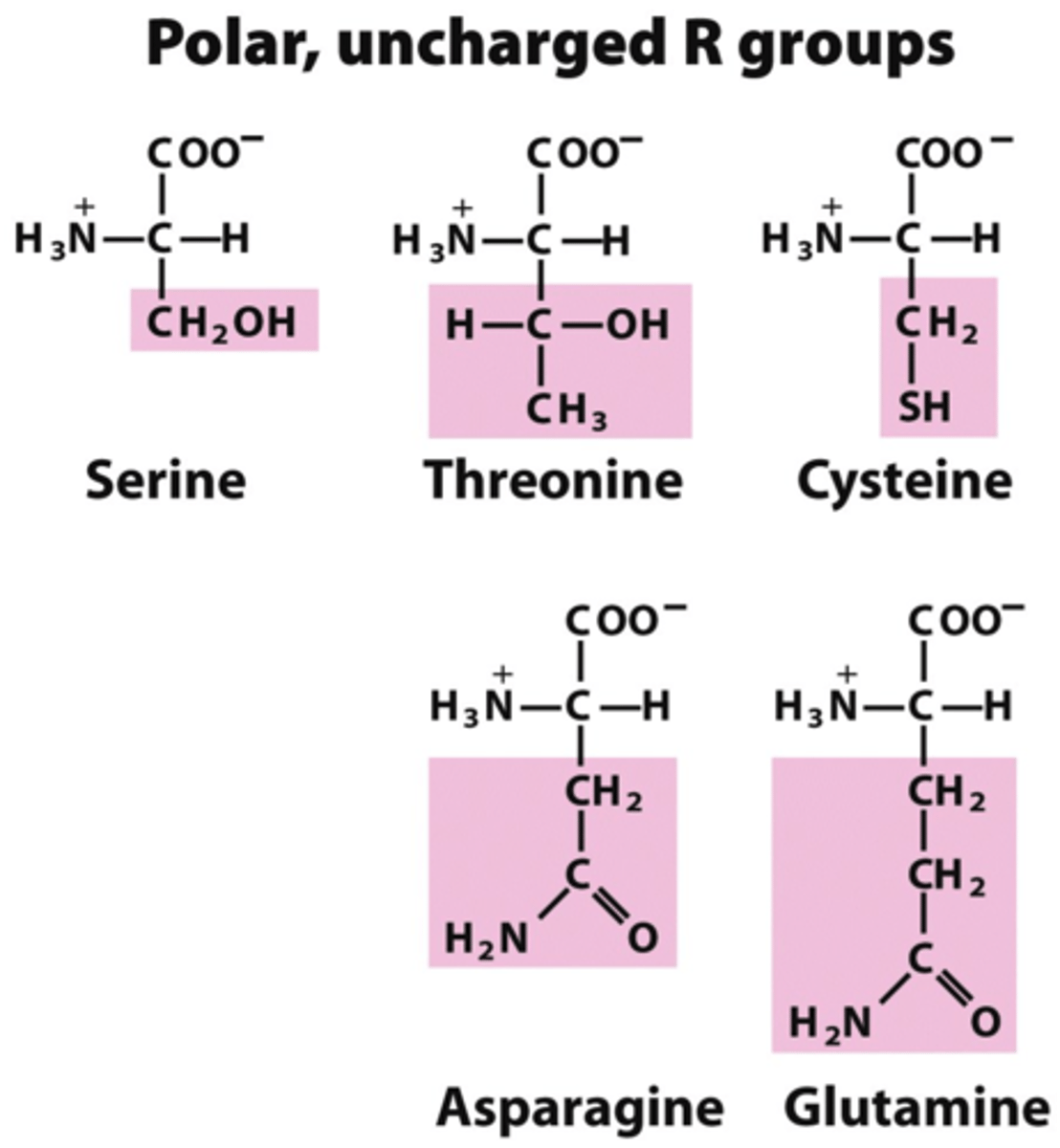
How do you know an amino acid is polar?
The R-group will have partial charges -OR- contain an uncharged O or N in the R-group
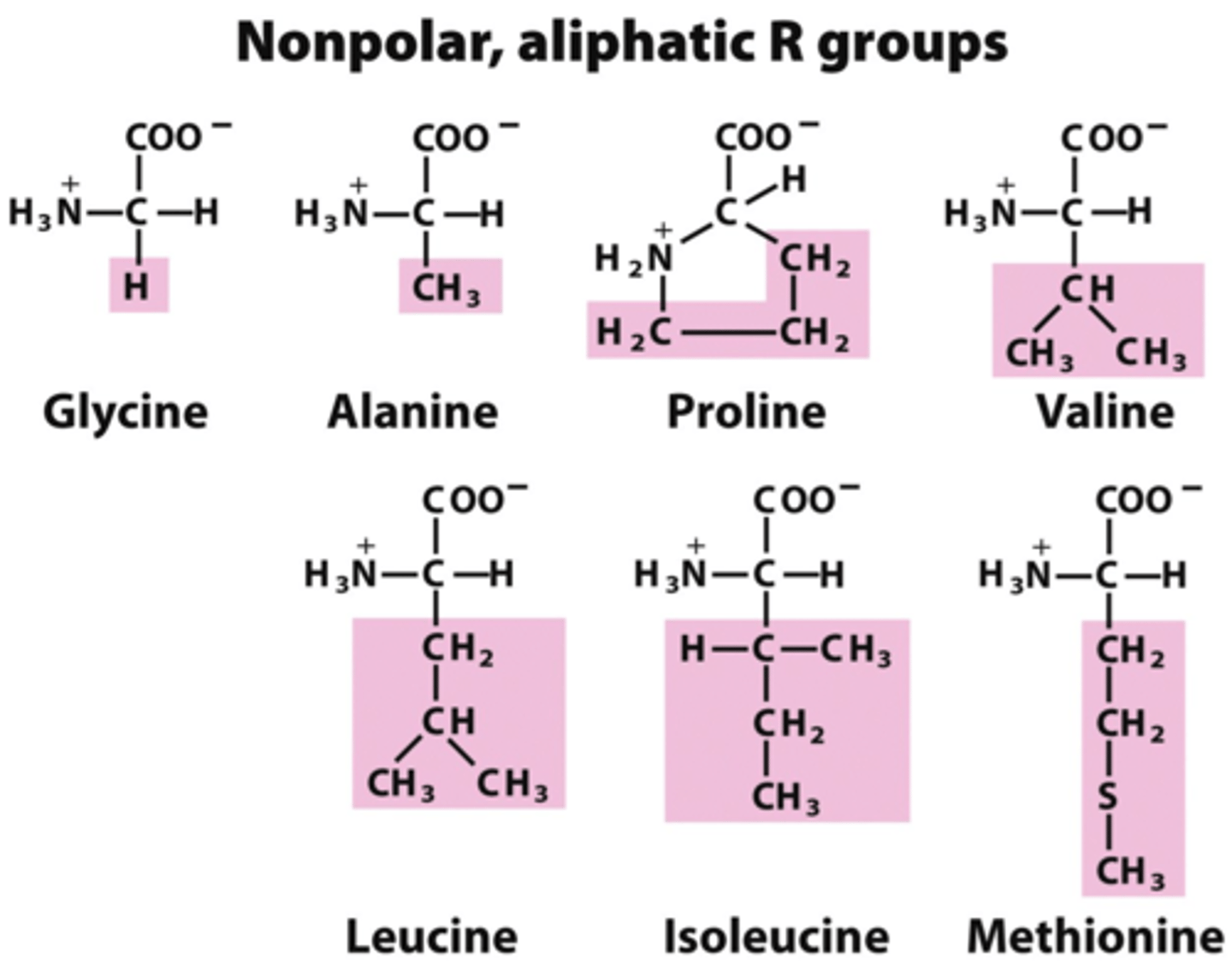
How do you know an amino acid is nonpolar?
The R-group will have no charge AND doesn't have an O or N in the R-group
What are key features of peptide bonds?
1. Directionality: N-terminous (amino group) synthasizes first to C-terminous (carboxyl group)
2. R-groups extend outwards and are involved in interactions
3. Paptide bonds can't rotate, but all others can. This allows proteins to fold into complex structures.
What are the levels of protein structure
1. Primary
2. Secondary
3. Tertiary
4. Quaternary
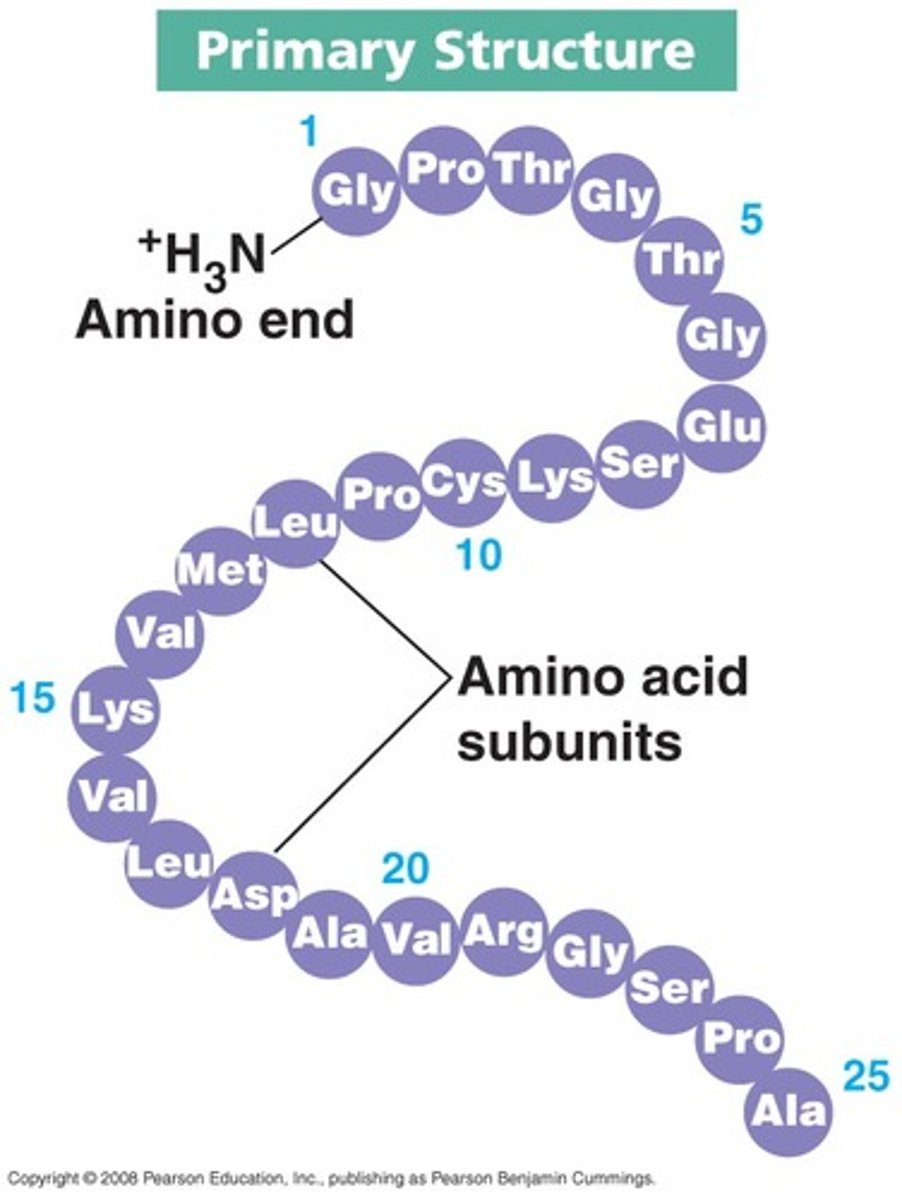
Describe the protein structure: Primary
--Unique sequence of amino acids
--Limitless number of possibilities
--Specific order of R-groups determines a protein's fold, properties, and functions
(Joined by peptide bond)
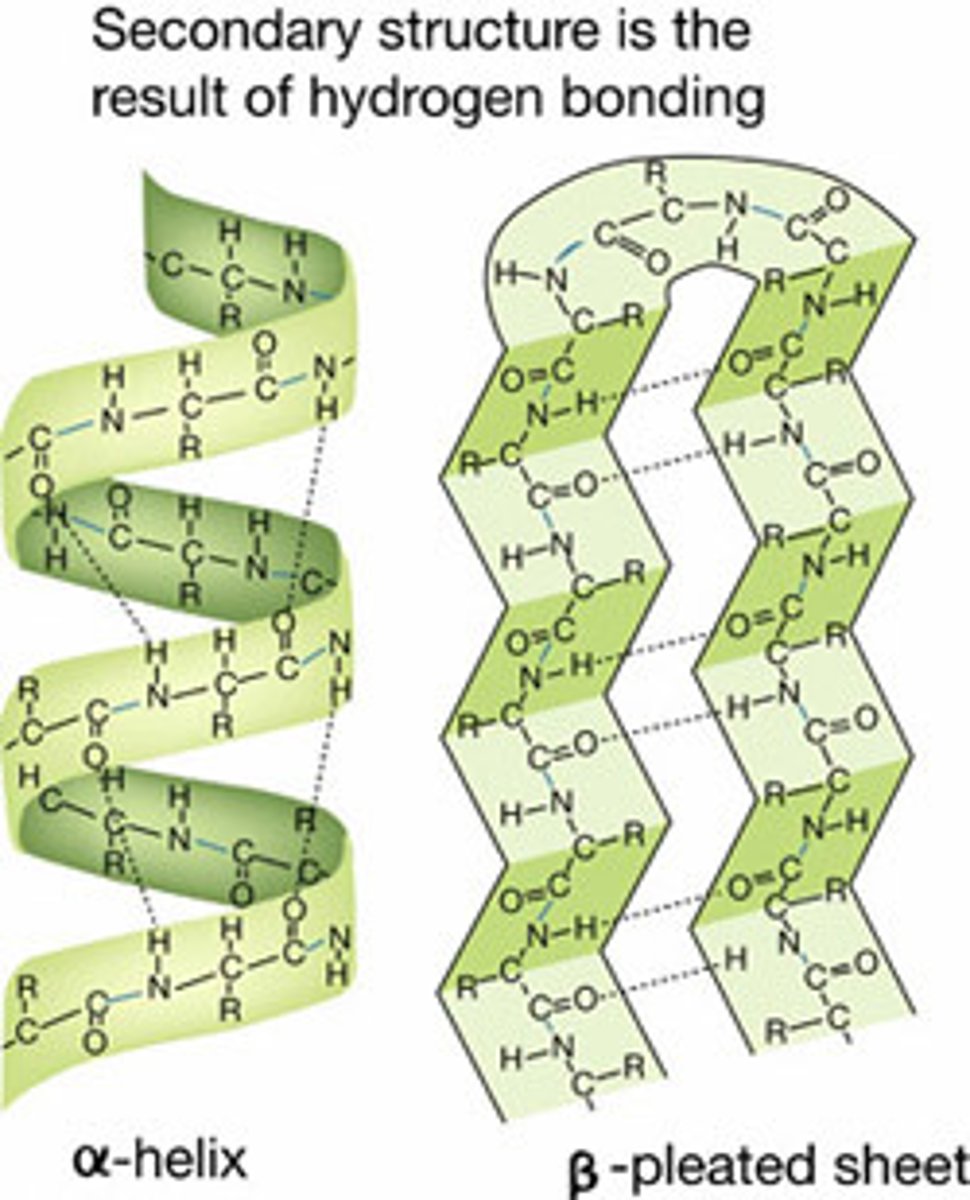
Describe the protein structure: Secondary
--Formed by hydrogen bonds between backbone of amino acids (one carbonyl group bonds to amino group of another amino acid)
--2 types: alpha-helices (spiral) and beta-pleated sheets (zig-zags)
(Joined by hydrogen bonds)
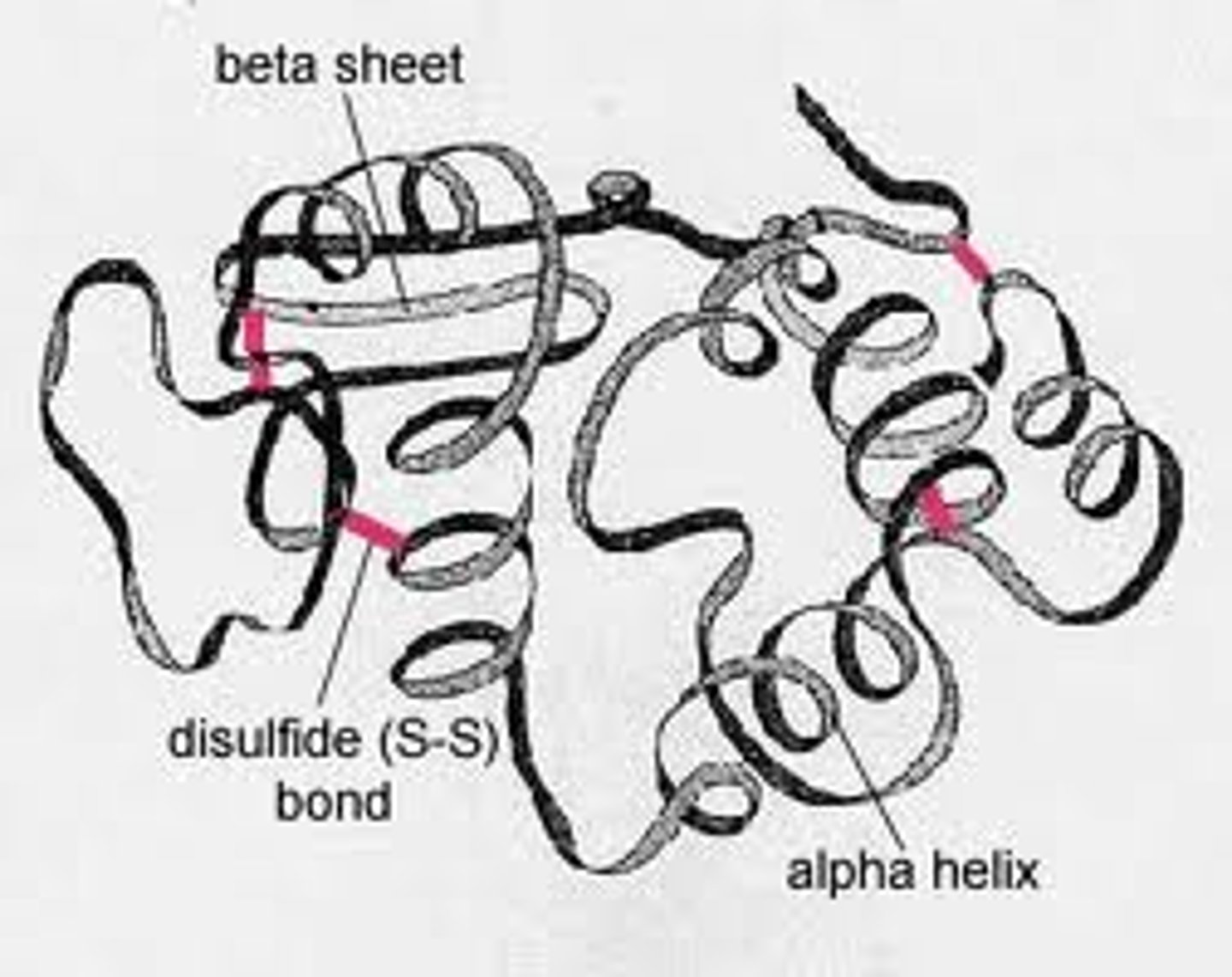
Describe the protein structure: Tertiary
--Distinctive 3D folded shape
--Interactions involving R-groups (typically long distance)
--Very diverse
(Joined by hydrogen bonds, hydrophobic interactions, disulfide bonds, and ionic interactions)
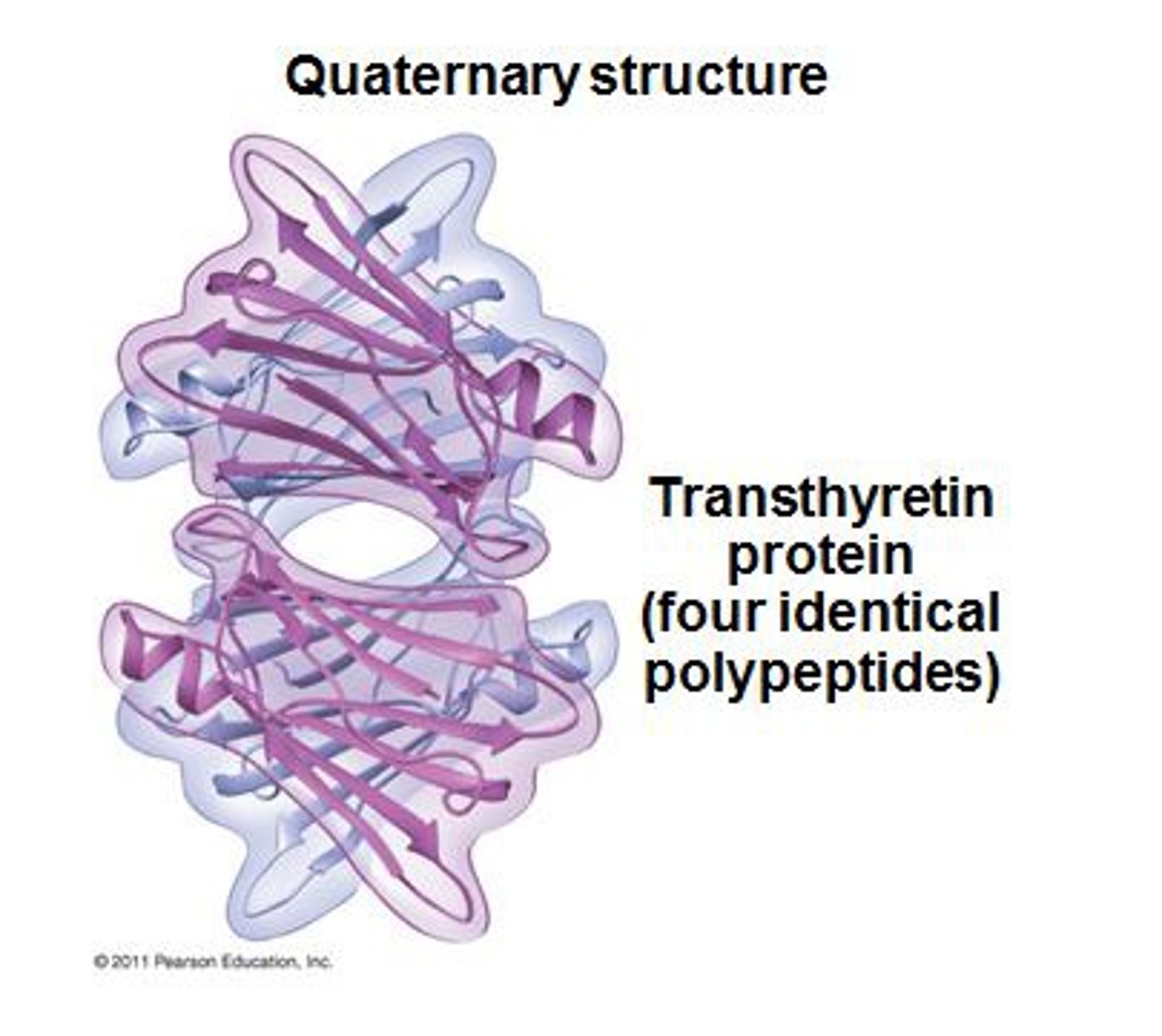
Describe the protein structure: Quaternary
--Multiple distinct polypeptides (subunits) interactingwith one another to form one functional protein
--Not all proteins have quaternary structure
(Joined by non-covalent interactions like hydrogen bonds, ionic bonds, hydrophobic interactions)
Why is primary structure so important to protein folding and function?
--Proteins build upon one another. One wrong fold effects all others in the series.
--Each fold is a conformation and some proteins only fold when needed for specific function (active conformation)
--A misfolding can result in infectious prions (can cause Alzheimer's)
What is protein denaturing?
A protein unraveling and losing its native shape due to an altered environment.
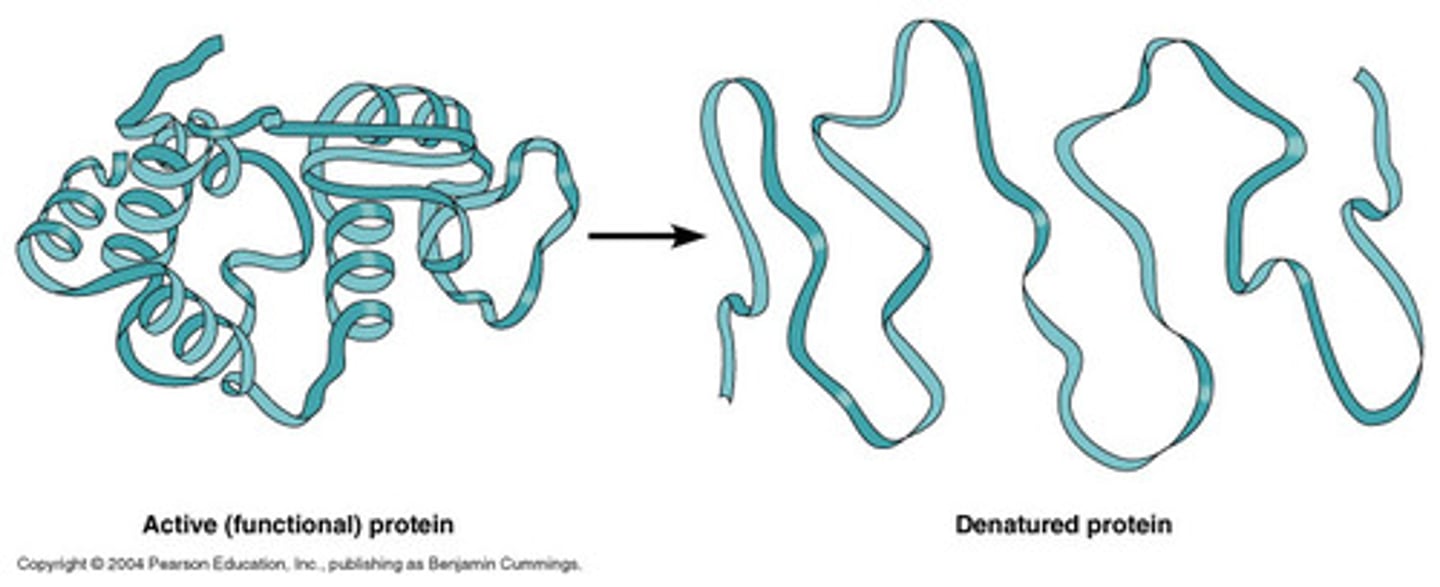
What are agents of protein denaturation?
1. Heat
2. pH extremes
3. Some salts
What are the most common functions of proteins?
--Catalyst (speed up chem reactions)
--Defense (antibodies attack pathogens)
--Movement (move cells/molecules within cells)
--Signaling (conveys signals between cells)
--Structure (shape cells and comprise body structures)
--Transport (allow molecules to enter and exit cells or carry them through body)
What are the basics of nucleic acids?
--Polymer: DNA or RNA / oligonucleotide (olig = "few")
--Monomer: nucleotide
--Linked by: phosphodiester bond
--Functions:
Information storage
Energy storage (ATP)
Chemical reactions (RNA only)
What are the components of a nuceotide?
1. Phosphate group (on 5' of sugar)
2. 5 Carbon sugar
3. Nitrous base (on 1' of sugar)
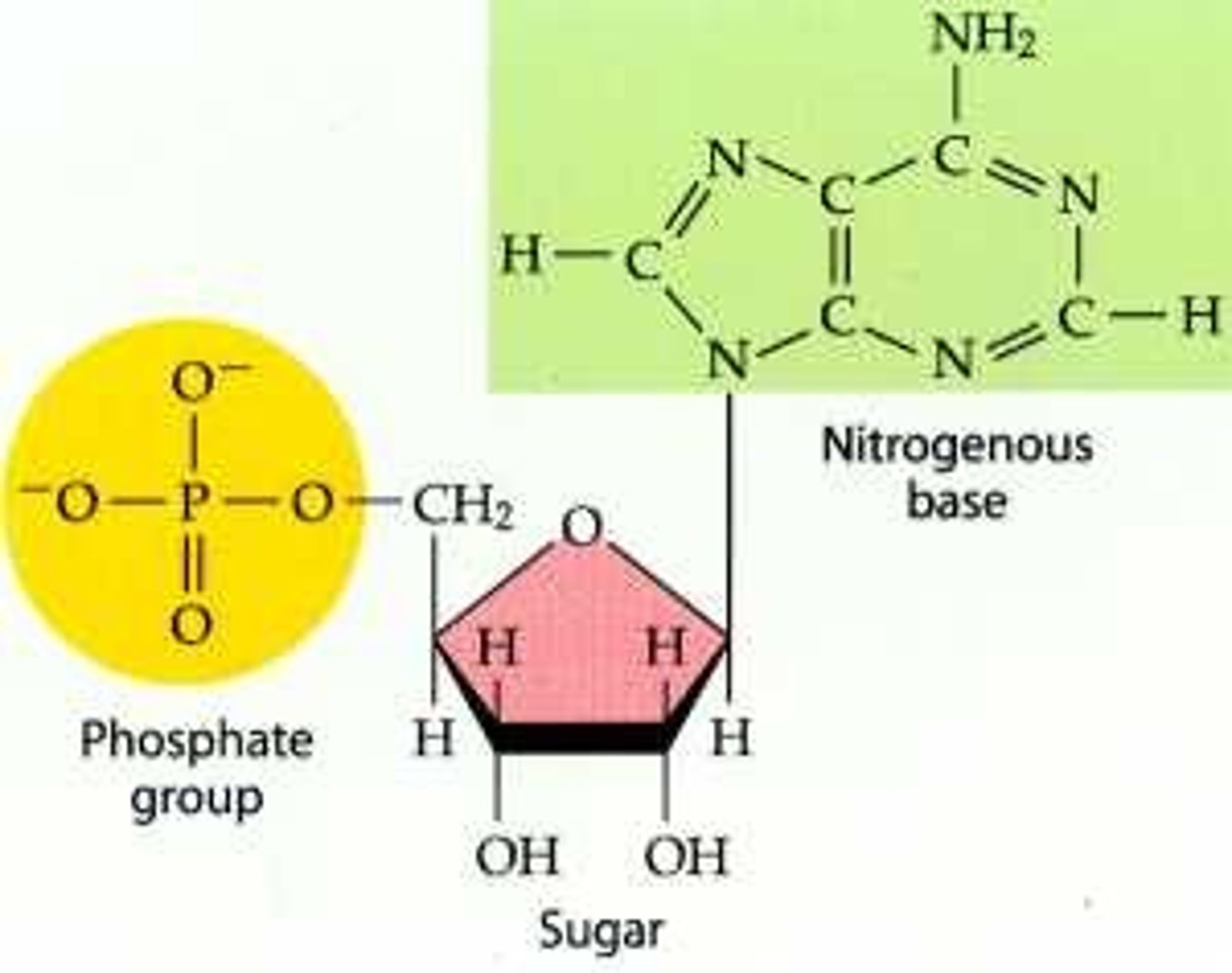
What is the difference between the sugars ribose and deoxyribose?
--Ribose has hydroxide (OH) on 2' of the 5 carbon sugar: this makes up RNA
--Deoxyribose has hydrogen (H) 2' of the 5 carbon sugar: this makes up DNA
Why is ATP a good source of chemical energy?
ATP is a good source of energy because it has a lot of potential energy stored when it keeps those negative phosphate groups together.
What is the difference between the base pairings in DNA vs RNA?
RNA has uracil (U) instead of thymine (T) like in DNA. Otherwise, they are the same.
How do the base pairings differ in DNA?
--A & T form 2 hydrogen bonds
--C & G form 3 hydrogen bonds
What is Chargaff's rule?
A=T and G=C
These sequences always form in a 1:1 ratio
This fact can be used to calculate all the base pairings in a strand of DNA.
How is the sugar-phosphate backbone formed?
--Phosphodiester linkages connect the hydroxide on the 5 carbon sugar to the hydroxide of the phosphate group
--DIRECTIONAL: 5' of phosphate always connects to 3' of 5 carbon sugar (5' --> 3')
What is the primary structure of DNA?
1. Strands held together by hydrogen bonds between one purine and one pyrimidine
2. Strands are antiparallel (match in opposite ways= 5' 3'--> 3' 5')
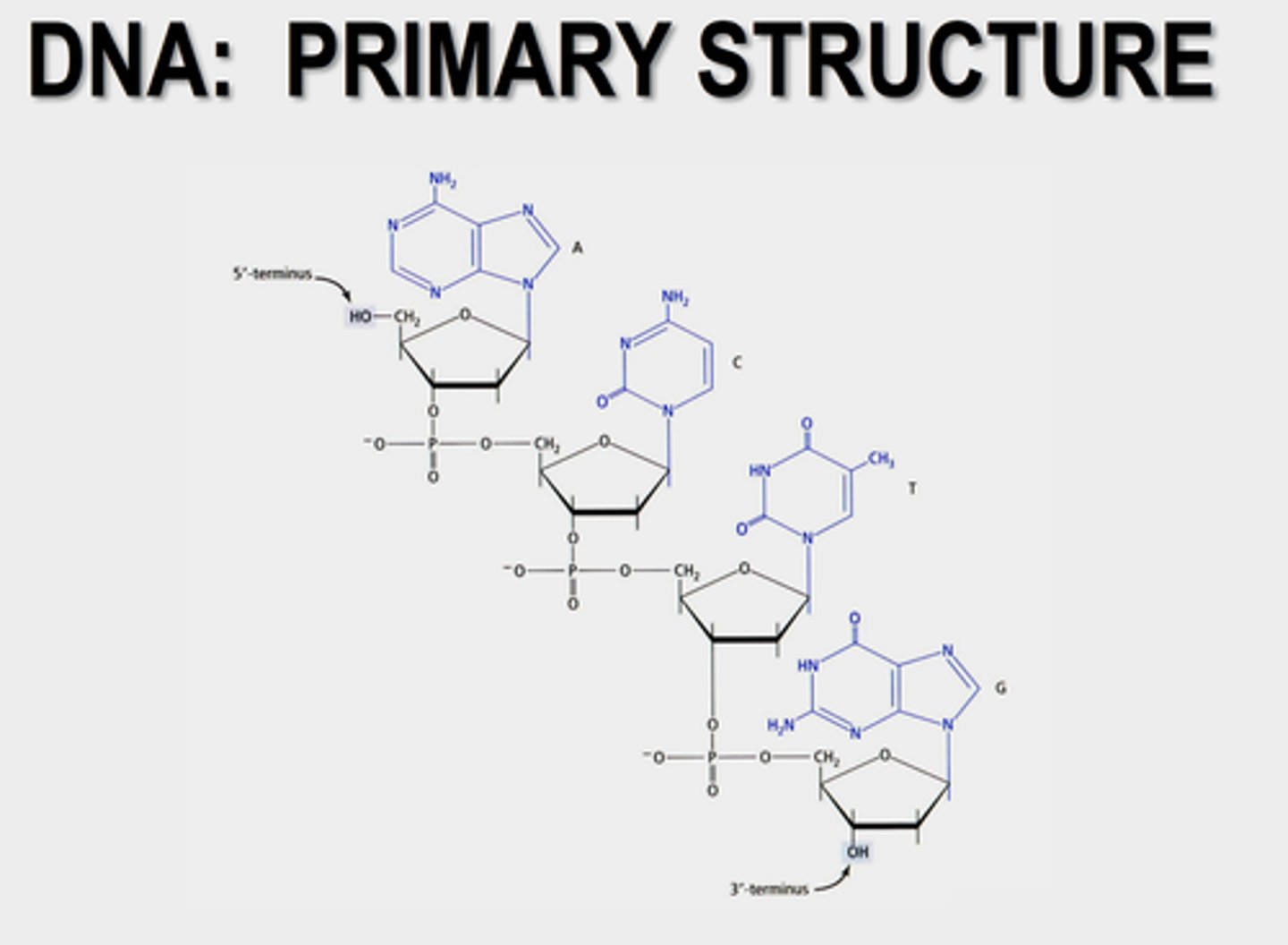
What is the secondary structure of DNA?
--Strands form a double helix (Sugar-phosphate backbone faces exterior and Nitrogenous bases face interior)
--Base pairing referred to as complementary (A=T & G=C)
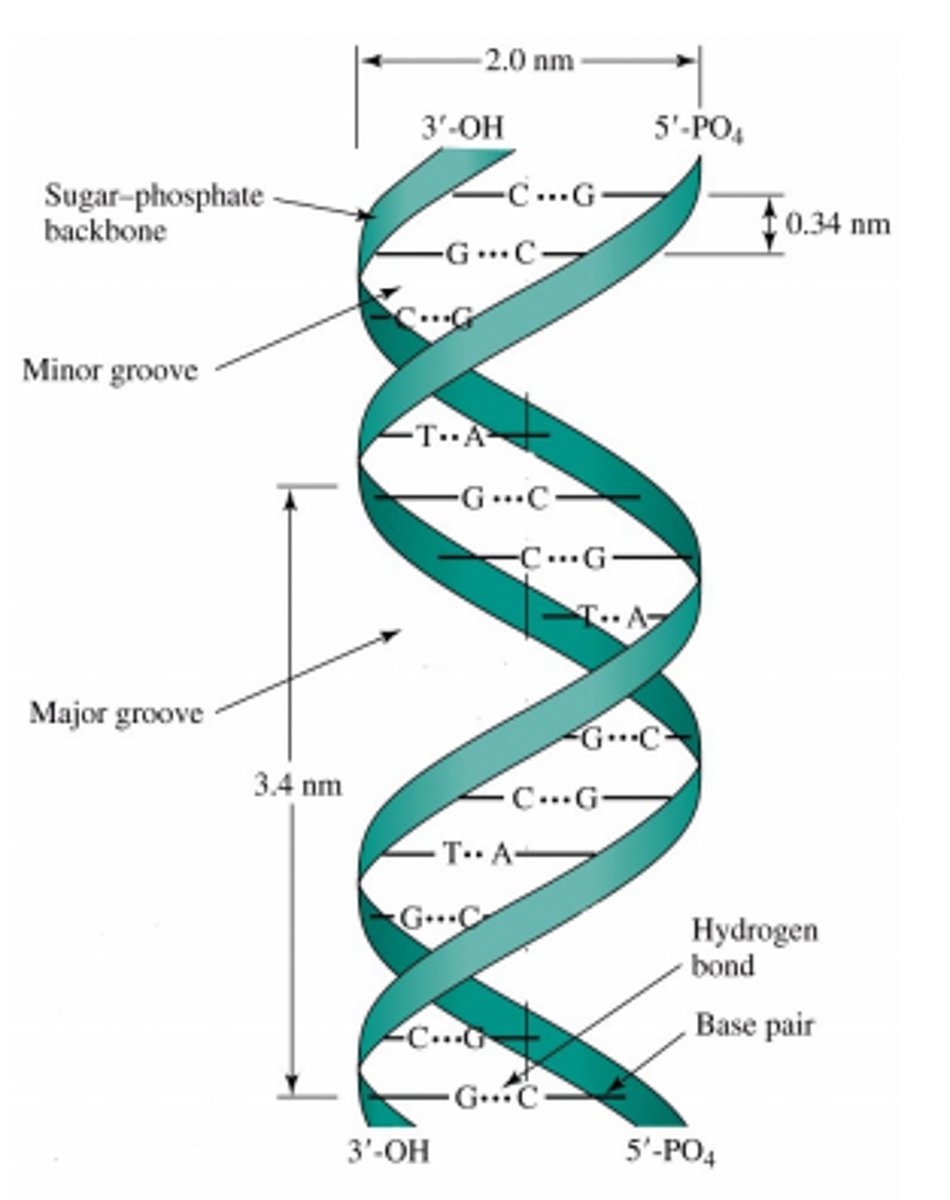
What is the tertiary structure of DNA
--Bacteria supercoil
--Eukarya produce nucleosomes
--Both forms provide compact structures for storage
**NOTE: Only DNA has tertiary structure
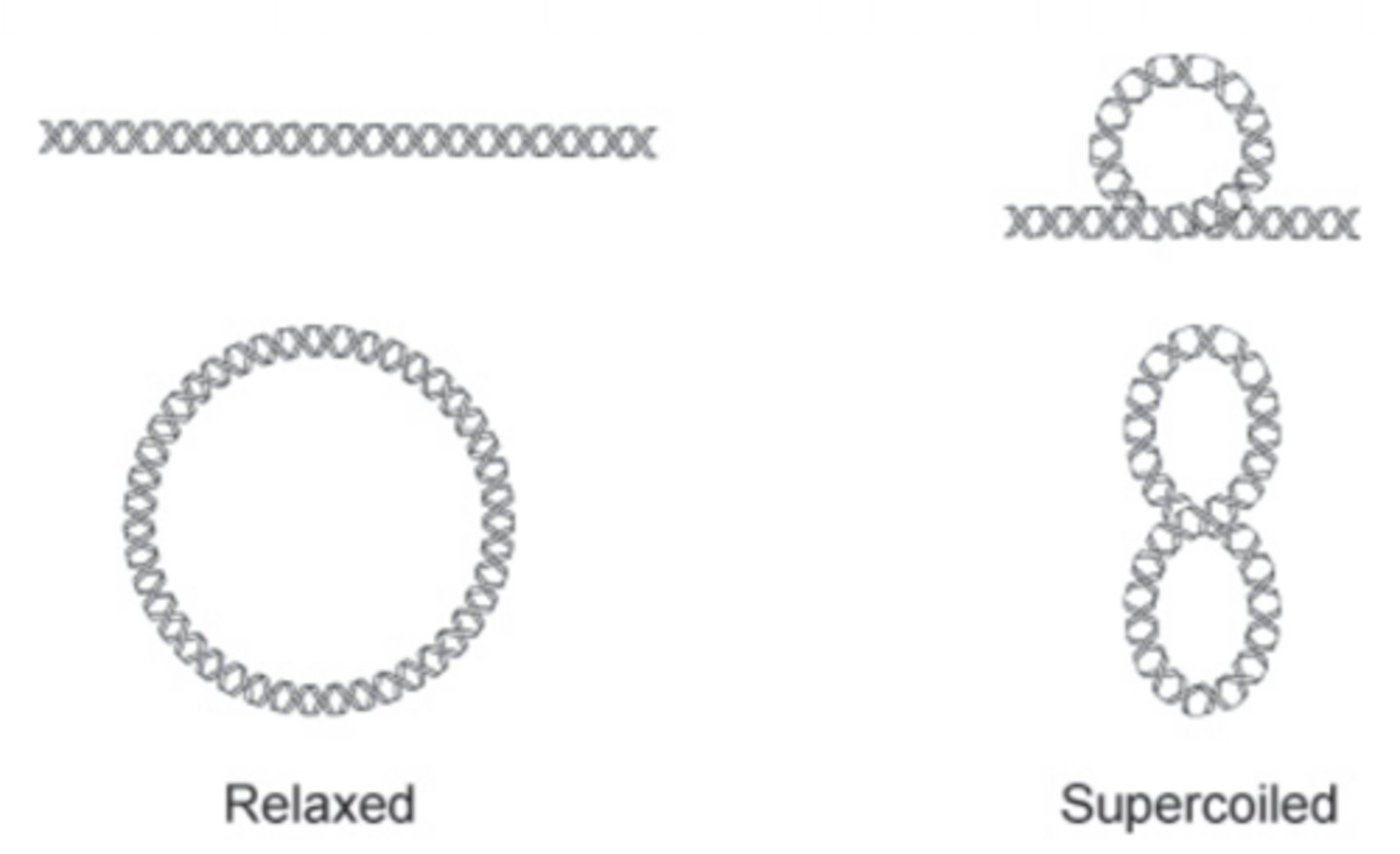
What is the function of DNA and why?
--Information Storage
BECAUSE:
1. Stable, unreactive structure
2. Serves as a template for its own synthesis