Immunity to Infection
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Leukocyte extravasation (diapedesis)
E, P-selectin expressed on the endothelial surface mediated sialyl lewisx on leukocytes, adhesion to leukocyte and allow leukocytes to roll along the vascular endothelial surface - weak adhesion
Start to adhere tightly via expression of LFA1 on leukocyte binding to ICAM 1 on the endothelial surface. The migration is regulated via the chemokine concentration of IL-8
What are found on all Gram (-ve) bacteria or Gram (+ve) bacteria?
LPS or
LTA lipoteichoic acid
Toxic shock syndrome toxins produced by S.aureus
Due to the release of superantigen leading matching between non-compatible MHCII and TcR leading to huge storm of IL-1, TNF-a, IL-2 leading to toxic shock
Changes to tissue - tissue damage when protect against bacteria
Neutrophil → platelet aggregating factor
Platelet aggregation (amplifier of permeablity) and fibrin deposition
PDGF from platelets stimulate phenotype modulation of smooth muscle→ remodelling and contraction
Microthrombi forms in small vessels which impair perfusion
Chemotaxis
ROI production - damage endothelium, exposing subendothelial collagen and tissue factor
Plasma leak into tissue → Edema, hypovolemia
Significant change in vascular permeability, loss of fluid into tissue, fall of blood pressure
Symptoms = fever, circulatory collapse, diffuse intravascular coagulation, haemorrhagic necrosis, blood leak out from vasculature
How is acute phase response generated?
Activated macrophage recognise bacteria via TLR
NF-kb transcription
IL-1, IL-6, TNF-a release
Signalling to liver hepatocytes to make acute phase proteins - mannan binding lectins, surfactans, C-reactive proteins (inflammation marker) opsonin effect, complement activation
What are the 3 main activities by neutrophils
Phagocytosis
ROS
NETS
Inhibitors of complement from bacteria (modulation or inhibition)
SCIN
SIC
CHIPs
SpA
SCIN: Staphylococcal complement inhibitor , binds to C3 convertases and inhibit C3 activation
SIC: Streptococcal inhibitor of complement binds C5b-7/8, prevent MAC formation
CHIPs: of Staph, aureus, binds to C5a receptor to inhibit C5a chemotactic signalling (release out, soluble)
SpA: of Staphylococcal, deposited on bacterial surface , binds Fc region of IgG which prevents opsonisation and classical pathway
Inhibition of complement via enzymatic degradation by bacteria
Pseudomonas protease
C5a peptidase
Pseudomonas elastase
Pseudomonas protease: cleaves C3, prevent C3b deposition
C5a peptidase: cleaves C5a and prevent neutrophil chemotaxis (bind directly to C5a NOT to the receptor)
Pseudomonas elastase: cleaves IgG and C1q, which prevents initiation of classical pathway
Role of LPS in immune evasion
Modification of LPS with sialic acid
Long side chains - O antigens
Modification of LPS with sialic acid - binds to Factor H, which is a complement regulator that breaks down C3b (in Neisseria)
Long side chains - O antigens - long side chain (in Pseudomonas) that makes a physical barrier so phagocytes cannot get to deposited C3b
Microbicidal mechanism of phagocytes: Oxygen dependent activity
Embeded on the phagosome - NADPH oxidase which pump oxygen through and makes superoxide inside the phagosome
ROS - O2-, H2O2 (from catalase)
Myeloperoxidase turns H2O2 into HClO
Catalase from lysosome
Microbicidal mechanism of phagocytes: nitric oxide pathway
IFNy - that is released as cytokines, binds to the receptor on the cell membrane of phagocytes - actvate JAK/STAT1 pathway
At the same time, TNF signalling on the membrane surface also enhances
Crosslinking of CD23 - FcrRII - crosslinking by immune complexes provides an additional activation signal
Boost NF-kB and AP-1 activity signalling
this induces nitric oxide synthase
NOS with tetrahydrobiopterin (cofactor)
L-arginine +O2+ NADPH → L-citrulline+ NO
NO reacts with O2- forms peroxynitrite ONOO- which is highly reactive
NO is toxic
Escape mechanisms from phaglysis by bacteria
Inhibits chemotaxis by Streptococcus o Staphylococcus
Capsule or outer coat prevents attachment and upatake
M. Tuberculosis - release of factors that blocks triggering and killing mechanism - block lysosome function and inhibit proton pump
M.tuberculosis, M.leprae - escape from phagosome
M,leprae - has phenolic glycolipid that scavenge free radicals
and lipoarabinomannan that blocks IFN-y signal
Counteraction of microbes to antibodies
Rapid cellular division
Intracellular location (TB)
Host-like coast
Antibdy specific protease
Antigenic variation → creates new epitope that antibody cannot bind
Which cytokines drift CD4+ differentiation into Th1 cells?
What are Th1 cell roles in response infection?
In an IFNy environment, Th1 differentation is induced
Th1 response s proinflammatory and stimulates macrophages activation and NK cells
Macrophages release IL-12 which supports Th1 activity
Leading to cytotoxic effector ADCC functions
Which cytokines drift CD4+ differentiation into Th2 cells?
What are Th2 cell roles in response infection?
In an IL-4,IL-5, and IL-10 environment, Th2 differentiation is induced
Stimulate B cells, mast cells and eosinophils
which generate a humoral response, ant-inflammatory
Skewing towards antibody production, allergic response
Limit macrophage activation
Low doses and oral route favours Th2 response
Example of a response that can be Th1 or Th2 - mycobacterium leprae
How is the Th1 response different to Th2 response?
Leprosy is caused by Mycobacterium leprae
Th1 response:
Vigorous host cellular response
Many immune cells in lesions
Tissue damage - Type IV hypersensitivity
Only few bacteria visibl microscopically
Disease progresses slowly and patient usually survive
Th2 response: Lepromatous form
Humoral response
Adequate antibody response but the antibody cannot reach the intracellular bacteria
MANY bacteria are found in lesions
Gross tissue destruction, fatal
Example of a disease that is both Th1 and Th2 - malaria at different stages
Caused by Plasmodium sp.
As sporozoites in blood: Th2 response - antibody production
As tissue schizonts in live: intracellular so now antibody response is useless: Th1 cytotoxic T cells, TNF, IL-1
As merozoites in blood: Th2 antibody production. But now the antigen is different so needs to mak new antibody
Asexual erythrocyte stage: both Th1/Th2 antibdy production, ROI, RNI, TNF
Note: specific antibody production always lag behind
Route of infection with Schistosoma (blood flukes) -parasitic flatworms
Symptoms
Schistosoma - larvae are released from water snail and penetrate skin
Migrate through blood and lung to hepatic portal vein (adult worm)
Egg movement to gut and urine bladder-released
Blockage of the lymphatic system, organ damage - can lead to schistosomiasis, abdominal ascites
Immune regulation and defence in schistosome infection
Firstly, the parasitic antigen cause mast cells to release histamine and heparin that are toxic to the larvae
Increases IL-4 release, which drives B IgE classwitch and also skewing towards Th2 response that release IL-5
Drives eosinophil -IgE binding activation which release MBP that damages the parasite
Released antibody IgG from B cell humoral production also recruits macrophages and neutrophils (that are part of the Th1 response to come in, which skews about to Th1 as TNFa and INFy are relased. They try to phagocytose the larvae but it’s too big so they become confused?
Still, neutrophil and macrophages release ROI and NO that causes damage to the larve
Neutrophil, macrophages and eosinophil release of toxicants = Antibody dependent mediated cytotoxicity
Immune response to viruses
What are the characteristics of virus
What is the early innate immune response
Adaptive immune response?
Viruses are obligate intracellular parasites that has high diversity due to high mutation rate
They are host specific - infection depends on specific host cell receptor
Innate immune response (early response)
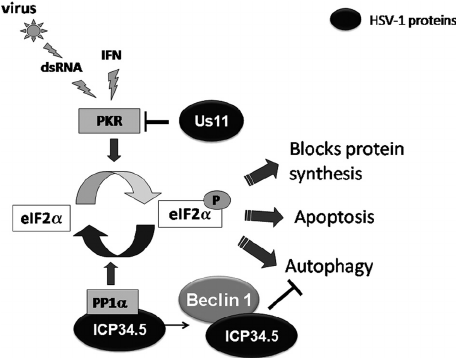
IFN stimulates inhibition of viral replication (IFNa and IFNb from infected cells signal to non-infected cell to regulate certain translation - TAP, MHC I, LMP - resistance to viral replication PKR, ribonuclease)
→ ribonuclease cleaves RNA, PKR upregulate eIF2
NK cells patrol and kill virally infected cells with low MHC I - ADCC
Complement can damag viral envelope
Adaptive immune response:
antibodies limit viral spread and reinfection; Ab mediated complement activation
CD8 T cells destroy virally infected cells → release IFN-y
Which group of virus does HIV belong to and what are the cellular targets?
Belongs to retrovirus - group: lentivirus, lentus =slow
Infect CD4 T cells, DC, macrophages
Cellular tropism of HIV - via which receptor does it gets into the cell?
Lymphocyte-tropic virus - X4 viruses - uses CXCR4, needs high level of CD4 on T cells
Macrophage-tropic virus - R5 viruses - uses CCR5 as co-receptor that is expressed on DC, M, and activated T cells
What are some treatments of HIV infection?
HAART: highly active antiretroviral therapy, protease inhibitor combined with 2 or more RT inhibitors
nucleoside analogues RT inhibitor (eg. AZT attached to the base which stops transcription
non-nucleoside analogues RT inhibitor - binds to RT enzyme
Typical course of untreated HIV infection
Primary (acute) infection is often asymptomatic or causes flu-like illness
HIV first infect DC which gets carried to the lymph node
T cells clustered with DC becomes highly activated - vigorous HIV replication
Drop in circulating CD4 T cells and activation of CD8+ cells
Asymptomatic phase
Cytotoxic CD8 cells and Th1 cells are responsible for decline of virus after initial infection
HIV generates humoral immune response (sero-conversion, important for diagnosis) that decrease the no virus but does not fully deplete it (HIV stay at low steady state)
Viral replication in lymphoid tissue (patients feel OK)
During the asymptomatic phase, no CD4 T cells gradually decline due to:
Direct viral killing
Increased susceptibility to apoptosis
Killing by CD8 CTL that recognise viral peptides
Throughout the pathway, CD4 drops slowly, once it drops to a critical number, the cell mediated immunity is lost, leading to the development of opportunistic infections
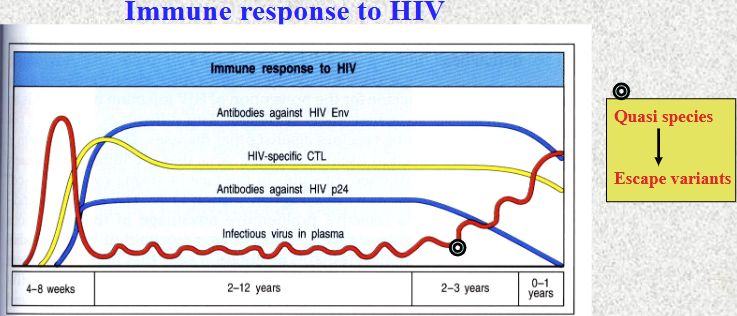
Quasi species - escape variants are formed from mutation of HIV once infected, worsen the prognosis because escape from the immune recognition - needs to go through the process again
HIV - escaping the immune system
Neutralising antibodies: control HIV particles in blood, but cannot eliminate virus altogether
High replication and mutation rate (quasi species develop into escape variants
Antigenic variation: immunogenic surface proteins (gp41 and gp120) develop new epitopes, not recognised by CTLs
Provirus hides inside the cell on host chromosome (latent infection)
HIV interferes with synthesis of MHC class I proteins
What is the influenza virus?
What is the role of its
Hemagglutinin (HA)
Neuraminidase (NA)
8 single stranded - negative sense virus
Hemagglutinin (HA): binds to sialic acid-containing receptors on epithelial cells of host
Neuraminidase (NA): cleaves sialic acid at the end of virus life cycle to allow mature virions to be released
Influenza virus
What is the innate response and adaptive response against this virus?
Innate response
Type 1 Interferon a and b to provide resistance against the virus
Viral replication/ cell lysis leads to production of IL-1, IL-6 and IL-8, TNF, IFNy
→ disease symptoms ( fever, cough, etc)
Adaptive response
viral clearance correlates with appearance of HA and NA specific - CD8 T cells
efficient clearance and protective immunity requires CD4T cell
Production of IgG and IgA primarily - neutralisation of HA
Antigen drift
Change in one specific viral genome
Neutralising antibody binds to the old virus so it cannot perform viral replication or enter the cell
Introduce point mutation to change the epitope, therefore the antibody cannot neutralise it and therefore can escape antibody binding
What are the subtypes of NA that infects human? What are the subtypes of HA that infects human?
What do they bind to?
H1, H2, H3
binds 2-6 sialic acid receptors
N1, N2
All subtypes are found in aquatic birds (natural reservoir for Influenza A virus)
Avian HA types binds to…
Pig HA types bind to …
Bind 2-3 sialic acid receptor
PIG Both 2-6 and 2-3 sialic receptor
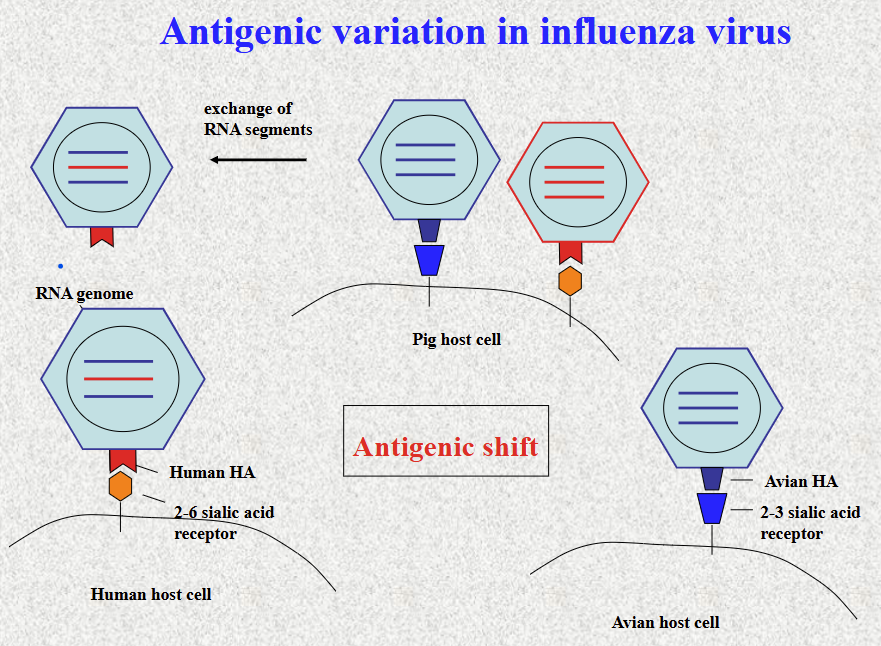
Antigenic shift
Two strands of influenza virus infect the same cell, so they exchange RNA segments (in PIG) , which leads to formation of a new strand that once could not bind human but now can bind human
genetic reassortment between human and avian forms in pig- emergence of new virus with high transmission
eg
H5N1 bird flu: highly pathogenic but low transmission - dont infect human (however pandemic possible if stran mutates and rearange with human chain)
H1N1: highly transmissive (binds human receptor) but not very pathgenic
Most protective method against Influenza…
vaccination! live attenuated
changes each year based on viral type