Mechanisms and Reactivity
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Describe the importance of the curly arrow
-Represents an electron pair
-A bond is formed between the atoms linked by the curly arrow
-A bond is broken by a curly arrow starting from a bond.
Describe the the first priority rule (Where electron flow starts) 4
The electron flow must start from an electron poor site
Negative charge
Lone pair
Double bond
Single bond
Describe the second priority rule (Where an electron flow moves) 5
The electron flow must move towards an electron poor site
Positive charge on a carbon (or H+) (as this means orbitals not full)
Atom adjacent to positive charge on a heteroatom (The adjacent atom has the LP donated by the hetero atom so we attack this and return the two electrons back to the hetero atom)
Towards a positive charge
Towards a partially positive charge
Partially positive charge at an alternating distance (Partial charges alternate down the backbone of a molecule, and this can mean it is possible to attack in some cases at more distant positions if for some reason attack at the other sites is prevented)
Describe the third priority rule (Where electron flow must end up)
Electrons like to end up on electronegative atoms
Ensure the correct number of bonds are present, e.g 4 bonds in carbon
3.Quenched by C+ or H+ with a new bond being formed to C/H
4.Breaking a pi bond to a heteroatom, leaving just a single bond
Breaking a sigma bond to a heteroatom, disconnecting it from the molecule
Describe the fourth priority rule (Charge)
Charge is conserved
Ensure charge is the same in every step of the mechanism
Describe the fifth priority rule (End of process)
Even once the processes is complete it may not be over
After completing the process the molecules may still be reactive and have reactive sites.
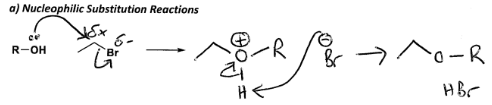
Draw out a nucleophilic sub reaction of R-OH and C2H5Br
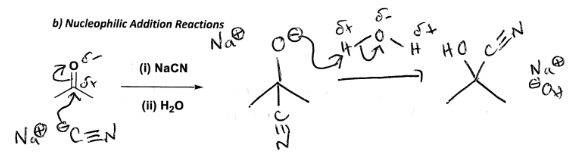
Draw out a nucleophilic addition reaction of C3H6O, NaCN, H2O
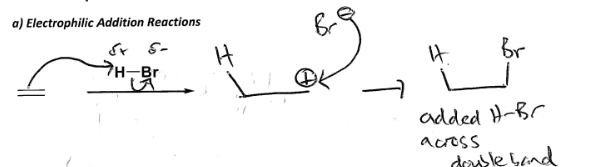
Draw out an electrophilic addition reaction between C2H4 and HBr
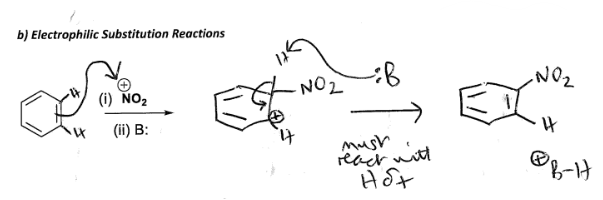
Draw an electrophilic substitution reaction with Cyclohexane, NO2, B:
Why do we need level two mechanistic priority rules
There may be a number of functional groups that would all be at the same level in terms of our simple priority rules. We need some more subtle rules to determine which one will preferentially react.
These will be based on the simple electronic effects, allowing us to predict which types of functional groups are most reactive
Define chemoselectivity
Chemoselectivity, when several different sites could react, one does so in preference. (one of the products is preferred)
Describe the first level two priority rule (where it starts)
So far this refers to just Negative charge > Lone pair > Double Bond
Considering more subtle differences,
we look at electronegativity as the more electronegative a molecule is, the easier it will give away electrons.
Mesomeric effects (resonance), greater resonance is worse as the electron’s position is shared so it will be less reactive
Sterics, more steric hindrance is bad for nucleophiles. Blocking the nucleophile'’ ability to approach and bond with an electrophilic centre due to the physical bulk of large atoms or groups
Describe the second level two priority rule (Where electrons end up)
2) The electrons must move somewhere electron poor
We use exactly the same approach, based on simple electronic effects, to predict the relative reactivity of electrophiles.
+ - I and + - M, are used to understand. Mesomeric effects are more important then I effects.
More +M means they are less reactive
More –I means they are more reactive
Where two different parts of a functional group could react, but only one of them does, we refer to this as regioselectivity.
Describe the third level two priority rule (Electrons end up)
We consider the electronic effects. We can apply the Principle of energetically accessible (or ‘stable’) intermediates. We choose the reaction pathway which has the most energetically accessible intermediates. This requires us to input the least energy.
How do we predict acidity and basicity
-The most acidic site will be the one that generates the most stable conjugate base
Negative charges should be on the most electronegative atoms
Products stabilised by resonance
-The most basic site in a molecule will be the one that has the greatest electron density and is most able to donate the electron density to a proton.
Lone pairs/negative charges should be localised
Less electronegative atoms can more easily donate their electron density.
How can we know wether to attack carbon or hydrogen based on the reagent
Depends on where the flow of electrons begins:
Nucleophile (Nu- ), A nucleophile is an electron rich species that will react with C+ or Cδ+
Base (B:), A base is an electron rich species that will react with H+ or Hδ+
How can we know what to attack based on the description of the element attracting the electron flow
-The place which attracts the electron flow is described as the electrophile (or acid)
Electrophile (E+ ), An electrophile is an electron poor atom (usually C + or Cδ+ ) that will react with a nucleophile (Nu- ).
Acid (H+ ), An acid is H+ or Hδ+ and will react with a base (B:).
Describe the orbital view of reactivity
What we are doing is finding the highest energy full orbital and saying we want it to react with the most energetically accessible, the lowest energy empty orbital. In order for these orbitals to react with one another, they need to constructively overlap.