Chapter 2: Amino Acids
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
Name all 20 amino acids and their abbreviations
Glycine (Gly, G)
Alanine (Ala, A)
Valine (Val, V)
Leucine (Leu, L)
Isoleucine (Ile, I)
Proline (Pro, P)
Methionine (Met, M)
Phenylalanine (Phe, F)
Tyrosine (Tyr, Y)
Tryptophan (Trp, W)
Serine (Ser, S)
Threonine (Thr, T)
Cysteine (Cys, C)
Asparagine (Asn, N)
Glutamine (Glen, Q)
Aspartic Acid/ Aspartate (Asp, D)
Glutamic Acid/Glutamate (Glu, E)
Lysine (Lys, K)
Arginine (Arg, R)
Histidine (His, H)
Describe Glycine
Gly, G
Non-polar (aliphatic, hydrophobic)
R= H
Only amino acid that is not chiral
Affected by hydrophobic effect
Only amino acid that has two protons bonded to alpha carbon
Key feature:
ammonia (NH3+)
Carboxygroup (COO-)
Hydrogen (H) on alpha carbon
Describe Alanine
Ala, A
Non-polar (aliphatic, hydrophobic)
Slightly hydrophobic
R= CH3
Key features
Ammonia (NH3+)
Carboxygroup (COO-)
Methyl (CH3)
Describe Valine
Val, V
R= CH3-CH-CH3
Non-polar (aliphatic, hydrophobic)
More hydrophobic, more bulky
Key Features:
Ammonia (NH3+)
Carboxy group (COO-)
Isopropyl group (C3H7) (CH3-CH-CH3)
Describe Leucine
Leu, L
non-polar (aliphatic, hydrophobic)
Very compact compared to valine
key features:
Ammonia (NH3+)
Carboxy group (COO-)
Isobutyl group (C4H9) (CH3 - CH - CH2 - CH3)
Describe Isoleucine
Ile, I
non-polar (aliphatic, hydrophobic)
Has TWO chiral centers
Alpha carbon
CH
Packing and hydrophobicity are affected
Key features:
ammonia (NH3+)
carboxyl group (COO-)
Isobutyl group (C4H9) (CH3-CH-CH2-CH3)
Describe Proline
Pro, P
Non-polar (aliphatic, hydrophobic)
A cyclic alpha-imino acid
Has two covalent interactions
Key features:
Ammonia (NH3+)
Carboxyl group (COO-)
Cyclic 5-membered rings with NH2+
Describe Methionine
Met, M
Non-polar (aliphatic, hydrophobic)
Doesn’t do anything since Sulfer (S) is not at the end of the chain
Key feature:
NH3
COO
CH3-S-CH2-CH2
Describe Phenylalanine
Phe, F
Aromatic, however absorbs the least amount of light compared to other aromatic amino acids
Very rigid and hydrophobic
Key features:
NH3
COO
CH2-6 carbon ring
Describe Tyrosine
Tyr, Y
Aromatic
The OH group on the top can hydrogen bond, as it is a hydrophilic tip
OH can have phosphate attached/replaced
Very weak acid
Phenolic hydroxyl pKa = 10.1
Key features
NH3
COO
CH2-Carbon ring-OH
Describe tryptophan
Trp, W
Aromatic, can absorb light very well and fluoresce
Rigid, biggest and largest amino acid
Has polarity
H on the NH is not stable, will not lose or gain protons
Key feature:
NH3
COO
Indole rung
Describe Serine
Ser, S
Polar, uncharged, hydrophilic
Alcohol pKa = 13.6
OH on the R group can be replaced by phosphate
Polar side changes, has polarity, can form H+ bonds
Key feature
NH3
COO
CH2OH
Describe Threonine
Thr, T
Polar, uncharged, hydrophilic
Alcohol pKa = 13.6
Not as polar than Serine
Key feature:
NH3
COO
H-C-OH
|
CH3
Describe Cysteine
Cys, C
Polar, uncharged, hydrophilic
Thiol pKa ~ 8.3
Can use H+ in oxidizing conditions
Two nearby Cys can form a disulfide bond in an oxidizing environment
Only Cysteine can do thiS
SH <—> S;
Disulfide bonds help stabilize protein structure
Brings two parts of proteins together (covalent bonds)
Key feature:
SH-CH2-
Describe Asparagine
Asn, N
Amide side-chain
Polar, uncharged, hydrophilic
Key features:
NH3
COO
O=C-NH2
|
CH2
Describe Glutamine
Glen, Q
Amide side-chain
Polar, uncharged, hydrophilic
Key feature:
NH3
COO
O=C-NH2
|
CH2
|
CH2
Aspartic Acid/Aspartate
Aspartic Acid (protonated)
Aspartate (deprotonated)
Asp, D
Negative at pH 7 (acidic)
Carboxyl pKa = 3.9
Most of the time it is aspartame due to the low pKa
Key feature:
O=C-O-
|
CH2
Glutamic Acid/Glutamate
Glutamic Acid (protonated)
Glutamate (deprotonated)
Glu, E
Negative at pH 7 (acidic)
Carboxylic pKa = 4.2
Key feature:
NH3
COO
O=C-O-
|
CH2
|
CH2
Describe Lysine
Lys, K
Positive at pH 7 (basic)
E-amino pKa = 10.5
Very weak acid
Protonated (positive charge)
Hydrophobic
Bulky
Key feature:
NH3
COO
NH3-CH2-CH2-CH2-CH2
Describe Arginine
Arg, R
Guanidino pKa = 12.5
NH2 can Gain or lose proton
Key feature:
NH2-C-NH-CH2-CH2-CH2
||
NH2+
Describe Histidine
His, H
Imidazole pKa = 6.0
Close to physiological pH = 7
N double bonded to NH is acidic, can have proton, can buffer, 10% would be charged
Key feature:
NH3
COO
imidazole side chain
When do acidic amino acids lose a proton and what are they?
Lose a proton before pH 7 and are anionic
When do basic amino acids bind to a H+ and what are they?
It can bind at whichever pH but around 7 best? They are cationic
What is molecular conformation?
Refers to differences in spatial arrangement of groups joined by covalent bonds due to bond rotation
no breaking/forming bonds, just rotating
What is molecular configuration?
Refers to isomers that can be interchanged only by breaking bonds
Arrangement of atom, must break and form bonds
What is an isomer?
Have the same molecular formula but a different arragement of the groups.
Ex. would be Leucine and isoleucine
What is a stereoisomer
When there are 2 possible arrangements of the 4 groups (not including H)
They are non-superimposable mirror images or enantiomers
In terms of enantiormers, what is D and L
D and L are defined as the absolute configuration and are derived from carbohydrate nomenclature
no matter how they are rotated, they cannot be superimposed in 3D
D = Right/dextro
L = Left/levo
Which isomeric form is most naturally occurring
The most naturally occuring amino acids are in the L-isomeric form
At what pH, what occurs to amino and carboxyl groups
At pH 7, the amino and carboxyl groups of amino acids are ionized.
Side chains in group A (non-polar), B (aromatic), C (polar) carry no net electric charge at pH 7 and remaain stationary in electric field.
What is a Zwitterion
It is a hybrid ion, meaning it carries both a positive and negative charge, it is electrically neutral.
They can act as both an acid or a base
If the zwitterion acts as an acid what happens?
Donates a proton so acid forms
If the zwitterion acts as a base what happens?
Accept proton, base is formed
how is pH important to an amino acid solution
By varying the pH of an amino acid solution, the charge on the amino acid can be changed
protons are added or removed
What can be said about amino acids and proteins in terms of pH
Amino acids and proteins are very sensitive to changes in pH. Outside the optimal pH range, structures may not function because charge is incorrect.
What happens to an amino acid under ionization?
Low pH (+)
CooH lose proton
Neutrality (zwitter)
Coo-
High pH (-)
NH3+ donates H+ and becomes NH2
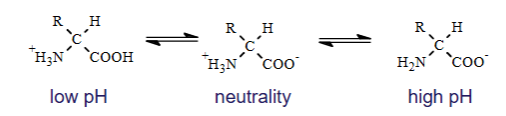
What happens when an amino acid with non-ionizable R-groups?
Since Glycine is non-ionizable the reaction will look like this
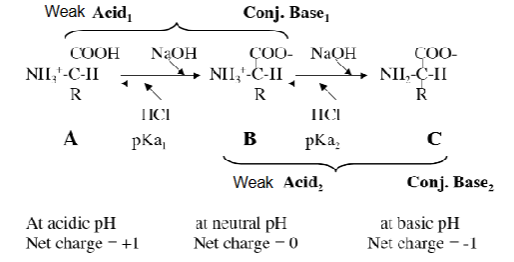
From the titration curve, describe it
It has two distinct stages, each corresponding to the removal of a proton
When titrating, what can be seen in the first stage
COOH converted to COO-
It is initially fully protonated. At the midpoint, equal amounts of 1st acid-base pair exist - this is the pKa for the COOH group
The removal of the proton continues with addition of NaOH. When the pH increases to 6, the protons from the COOH group are lost completely
When titrating, describe the second stage
NH3+ is converted to NH2
Midpoint: the pH = pKa of the NH3+ group. Equal amounts of the acid-base pair exist. Then, at pH 12 the titration is complete (the last plateau, as the pH fo the NaOH itself is approached).
What are 3 key information gained from titration
Pka values of the two ionizable groups determiend
SHows the two buffering regions; one around the first pKa and one around the second pKa
The curve shows the relationship between the charge of the species and the pH. At pH 6, the point of inflection between the two stages, the amino acid is present as its dipolar or zwitterion. ie. ionized but no net charge. This pH is called the ISOELECTRIC POINT or pI. It is the arithmetic mean of the 2 pKa values
For all 20 amino acids, what is the pKA values for alpha-COOH and alpha-NH3+
COOH: Fall in the range of 1.7 - 2.6
NH3+: 8.9 - 10.8
What can be said about non-ionizable R-groups titration curve and ionizable R group titration curves?
All amino acids with non-ionable R groups will have titration curves like Glycine (2 peaks, very simple) 13 of the 20 common amino acids fall into this category
An amino acid with an ionizable R group has more complex titration curve. The different R groups ionzie at acidic, neutral or basic pHs (7 in total)