Chemistry Final Study Guide
1/54
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
Name 3 ways a chemistry student should dress in lab
close-toed shoes, long sleeves, goggles, apron, no loose clothing, hair up
Name 3 pieces of safety equipment, their location and when it would be appropriate to use each
Fire blanket- Front of classroom, to put out fires. Emergency shower- back of classroom, chemical burns. Eyewash- next to sink and goggles cabinet, to wash eyes.
What is the density formula?
D= Mass
Volume
What is water’s density?
Why is it important to know?
1g/ 1mL
You can convert between grams and mL easily.
How to find volume of a cube?
(side cubed) s3
How to find volume of an irregular shaped object?
Water displacement
What are the 7 diatomic elements?
Hydrogen, Oxygen, Nitrogen, Fluorine, Chlorine, Bromine, Iodine
What is the fourth state of matter?
Plasma
What defines matter?
Anything that takes up space and has mass.

What kind of mixture is this?
homogenous

What kind of mixture is this?
heterogenous
How do you know if a chemical reactions occured?
Changes on a molecular level. (Gives off smell, temperature change, changes color)
Solid to liquid?
Melting
Liquid to solid?
Freezing
Liquid to gas?
Vaporization
Gas to liquid?
Condensation
Solid to Gas?
Sublimation
Gas to solid
Deposition
what is Boyle’s law?
(Pressure and Volume are inversely proportional) P1V1=P2V2
what is Charles’ law?
(Volume and Temperature are proportional)
V1 / T1 = V2/ T2
what is Gay-Lussac’s law?
(Pressure and Temperature are proportional)
P1 / T1 = P2/ T2
What is STP?
1 ATM and 273.15K (aka 0 degrees)
1 mole gas = 22.4L
What is the first prefix for covalent bonds?
Mono
What is the 2nd prefix for covalent bonds?
di
What is the 3rd prefix for covalent bonds?
tri
What is the 4th prefix for covalent bonds?
Tetra
What is the 5th prefix for covalent bonds?
Penta
What is the 6th prefix for covalent bonds?
Hexa
What is the 7th prefix for covalent bonds?
Hepta
What is the 8th prefix for covalent bonds?
Oct
What is the 9th prefix for covalent bonds?
Non
What is the 10th prefix for covalent bonds?
Deca
What is the name for group 1 on the periodic table?
Alkali metals
What is the name for group 2 on the periodic table?
Alkaline Earth metals
What is the name for group 7 on the periodic table?
Halogens
What is the name for group 8 on the periodic table?
Noble gas
Properties of metals
Lose elections to non metals, form a cation, conduct energy, malleable, magnetic, shiny
Properties of nonmetals
Gain electrons from metals, form anions, provide insulation, brittle (can’t be bent)
The most electronegative element?
Fluorine, because It is the smallest element that isn’t a noble gas.(Holds electrons closest to itself than another other element)
How do ionic bonds occur
Between metal- nonmetal. Transfer of electrons between atoms. Bonded by electrostatic attraction.
How does a covalent bond form?
Between nonmetal-nonmetal. Electrons are shared between atoms.
Properties of ionic bonds
Dissociate into ions in contact with water, Make salts. No double and triple bonds.
Properties of covalent bonds
Can make single, double and triple bonds. Can be any state of matter.
When is hydrogen a metal? A nonmetal?
Metal: written first. Nonmetal: written second.
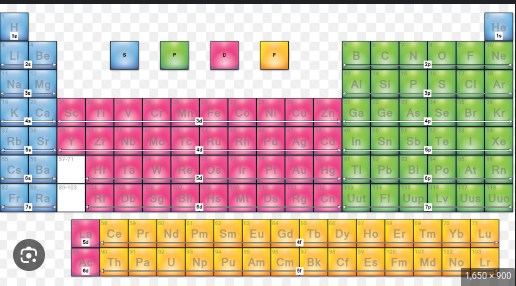
What orbital is colored yellow?
F orbital

What kind of chemical reaction is this?
single replacement

What kind of chemical reaction is this?
double replacement
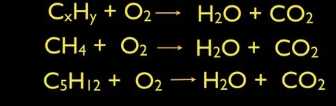
What kind of reaction are these?
Combustion
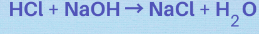
What kind of reaction is this?
acid-base neutralization
What are some common acids and bases?
NaOH (sodium hydroxide) HCl (Hydrochloride)
Does an acid or base turn blue litmus paper red?
Acid
Does an acid or base turn red litmus paper blue?
Base
Do endothermic reactions release heat or absorb heat?
absorb heat
What is standard psi?
14.7
Do exothermic reactions release or absorb heat?
release heat