3.1.4.3 - Hess's law
0.0(0)
Card Sorting
1/5
Earn XP
Description and Tags
Last updated 7:32 PM on 1/19/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
1
New cards
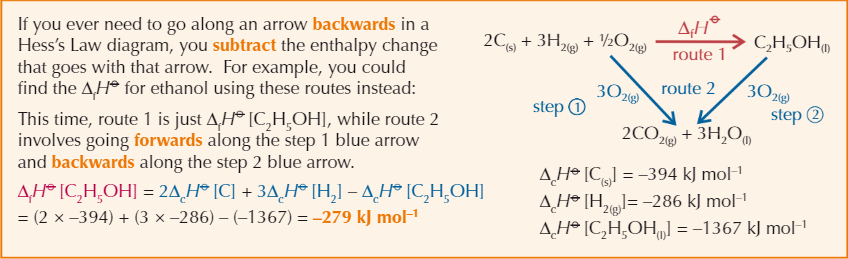
Hess’s Law
Total enthalpy change of reaction is independent of the route taken
2
New cards
Hess’s Law example

3
New cards
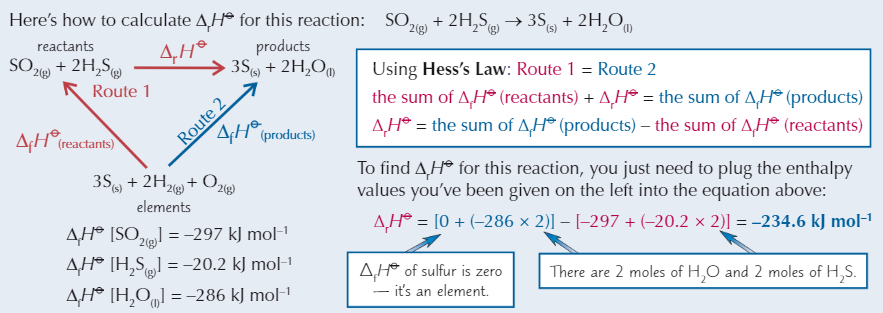
Calculating enthalpy change using enthalpy of formation
Need to know ΔfH⦵ of all reactants + products that are compounds
4
New cards
Hess’s Law equation
ΔrH⦵ = sum of ΔfH⦵ products - sum of ΔfH⦵ reactants
5
New cards
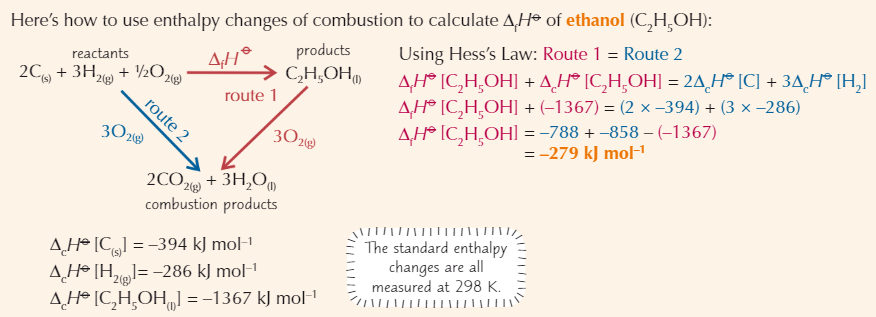
Calculating enthalpy change using enthalpy of combustion
6
New cards
Hess’s Law diagram with backwards arrow example