Lecture 21: Viral Genetics and Gene Therapy
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Virus
A single particle (virion) that consists of a protective outer layer (capsid) encasing a DNA or RNA genome which can be single-stranded or double-stranded
May also have a viral envelope, a second layer that helps virions attach to host cells
Viral genome contains structural genes and non-structural genes
Structural genes — needed to assemble the virus (capsid genes and envelope genes)
Non-structural genes — needed for replication

Structural genes
Genes needed to assemble the virus, including capsid genes and envelope genes
Non-structural genes
Genes needed for replication of the virus
Describe the general viral life cycle.
Virus binds to a receptor protein on the host cell membrane and is taken into the cell, usually through an endosome
Virus releases its genome into the host cell and the viral genome is transported to the nucleus
Virus uses its own viral replication genes as well as the eukaryotic host cell replication machinery to replicate and transcribe viral proteins → host cell makes copies of the virion’s capsid and envelope components
Virus assembles new virions with host cell-synthesized capsid and/or envelope proteins
The newly assembled viruses are released out of the cell or bud out of the cell

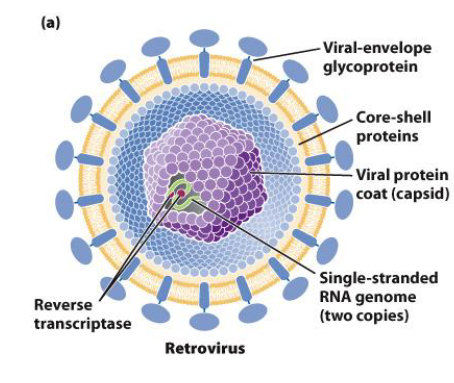
Retrovirus
A type of RNA virus that uses reverse transcription to convert its viral RNA into viral DNA for incorporation into the host DNA genome
Contain a viral envelope coated with glycoprotein
Contain a viral capsid protein coat
Have a single-stranded RNA genome that contains reverse transcriptase and integrase
Describe the steps of retrovirus replication.
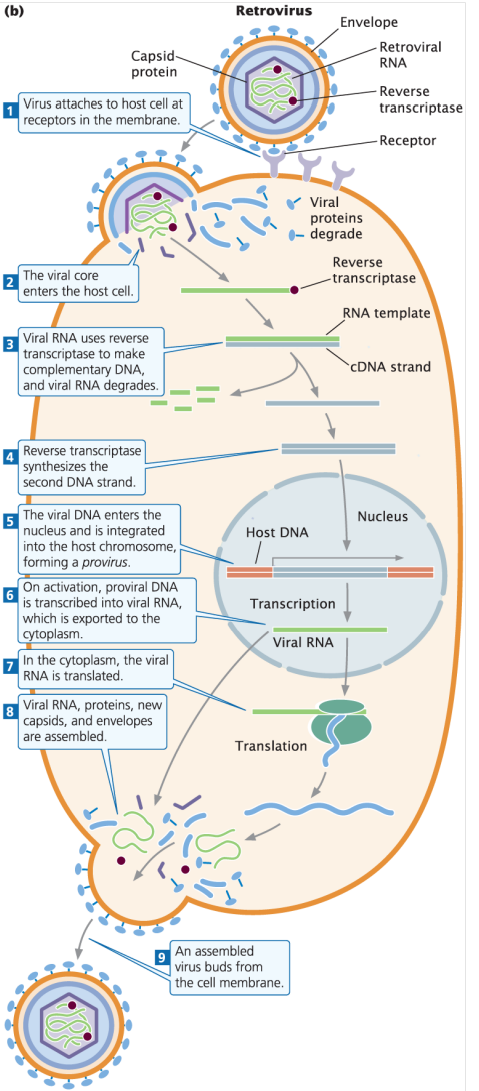
Retrovirus attaches to host cell at receptors on the host cell membrane
Retrovirus core containing the viral RNA enters the host cell, while viral proteins degrade
Viral RNA uses reverse transcriptase to make cDNA (complementary DNA) → viral RNA degrades after fulfilling its function
Reverse transcriptase synthesizes the second cDNA strand
Viral DNA enters the nucleus and is integrated into the host genome with the help of integrase, forming a provirus
Upon activation, proviral DNA is transcribed into viral RNA → viral RNA is exported to the cytoplasm and translated
Viral RNA, viral proteins, capsid coats, and viral envelopes are assembled in the cytoplasm before exiting the host cell
Provirus
When a retrovirus incorporates its viral DNA (after reverse transcription) into the host genome with the help of integrase
Integrase
An enzyme that facilitates integration of retroviral DNA into the host cell’s genome
Which of the following describes a mutation in a eukaryotic cell that would inhibit uptake/infection of the virus?
A) A nonsense mutation in reverse transcriptase
B) A silent mutation in the receptor that binds the virus
C) A frameshift mutation in a secretory protein necessary for viral budding
D) A transition mutation in the promoter consensus sequence necessary for receptor gene transcription
E) Both B and D
Answer: D) A transition mutation in the promoter consensus sequence necessary for receptor gene transcription
Which of the following describes a mutation that would inhibit a retrovirus, but not the general viral life cycle?
A) A missense mutation in reverse transcriptase that causes a neutral mutation
B) An in-frame deletion of an amino acid in the integrase enzymatic site
C) A frameshift mutation in a secretory protein necessary for viral budding
D) A transition mutation in the promoter consensus sequence necessary for receptor gene transcription
E) All of the above
Answer: B) An in-frame deletion of an amino acid in the integrase enzymatic site
Describe the two ways that retroviral infection can cause cancer.
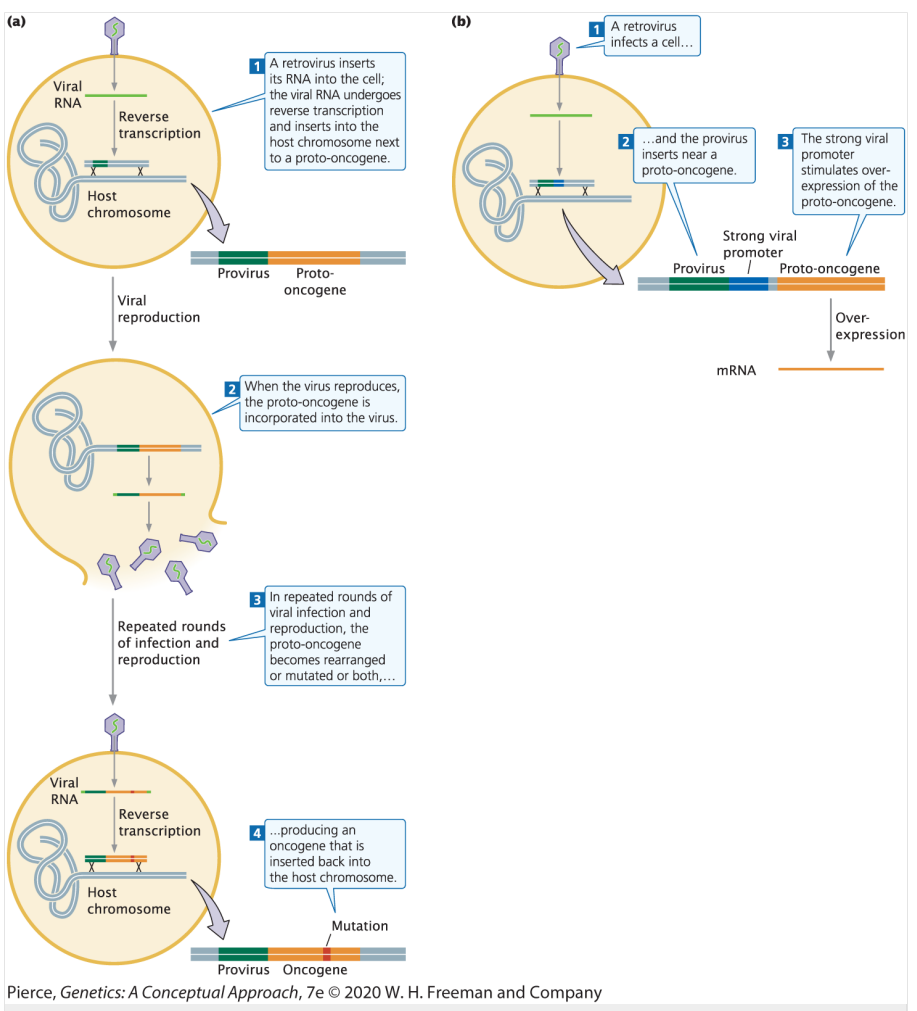
Method 1: Proto-oncogene mutation to oncogene
Retrovirus inserts viral RNA into the cell → retroviral RNA undergoes reverse transcription and inserts into the host chromosome next to a proto-oncogene
When the virus reproduces, the proto-oncogene is incorporated into the virus
After repeated rounds of viral infection and reproduction, the proto-oncogene is rearranged and mutated so that it becomes an oncogene that is inserted back into the host chromosome
Method 2: Overstimulation of proto-oncogene promoter
Retrovirus infects a cell and the provirus inserts near a proto-oncogene
The strong viral promoter stimulates overexpression of the proto-oncogene
Ex. Human T-cell leukemia virus type 1 (HTLV-1) causes a form of adult T-cell leukemia/lymphoma
Tumor suppressor genes
Genes that inhibit cell division in the presence of DNA damage or lack of resources
Proto-oncogenes
Genes that stimulate normal cell division
Oncogenes
Mutated, dominant-acting, stimulatory genes that cause uncontrolled cell division leading to cancer
Influenza virus
A RNA virus that has 3 main types: influenza A, influenza B, and influenza C
Most cases of influenza A are divided into subtypes based on glycoprotein expression → hemagglutinin (H) or neuraminidase (N)
Responsible for 1918 Spanish flu pandemic, Asian flu, Hong Kong flu, and 2009 H1N1 swine flu
How are new strains of influenza virus created?
Antigenic shift — when a cell is infected by multiple viruses, those multiple viral genomes can recombine to create new types of viruses
Reassortment of RNA molecules from different strains can create new strains
Antigenic shift is the reason why we need to get new flu vaccines every year → evaluate which strain will be dominant when multiple strains are present in the same cell, and generate a new vaccine

Adenoviruses
Medium-sized, non-enveloped virus containing a double-stranded DNA genome
Causative agent of mild cold or flu-like illnesses
Adeno-associated virus (AAV) vectors or cassettes are the most commonly used viral vector for gene therapy
How do scientists use adeno-associated virus (AAV) vectors for gene therapy?
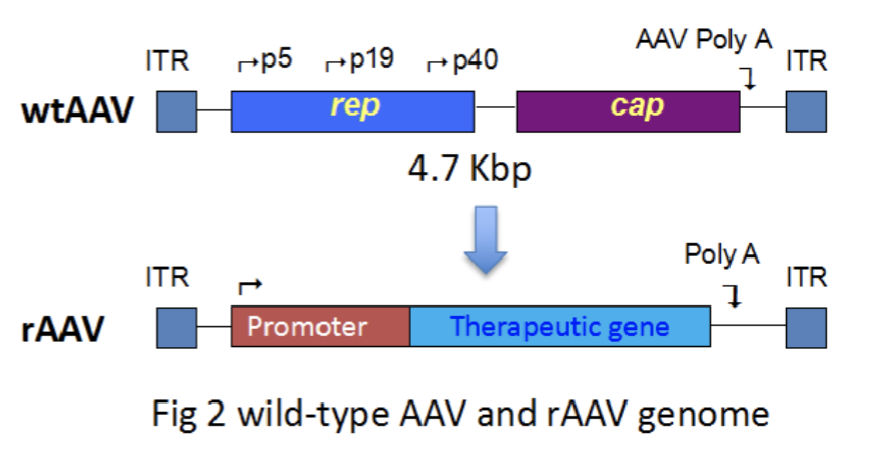
Wild-type AAV genome contains replication genes and capsid genes between inverted terminal repeats (ITRs)
Scientists create a recombinant AAV by removing the replication genes and capsid genes and cloning in a promoter upstream of a therapeutic gene of interest
The replication and capsid genes are removed so that the therapeutic AAV vectors does not replicate uncontrollably within the patient
Once the virion is made, it cannot replicate itself
Gene therapy
An experimental technique that uses genes to treat or prevent disease — the idea is to insert a gene into a patient’s cells instead of using drugs or surgery
Describe how to clone DNA for AAV-based gene therapy.
Isolate genomic DNA from cells that have your DNA sequence or gene of interest.
Use PCR to amplify the DNA with sequence-specific primers (forward and reverse) that contain restriction enzyme sites
Cut the amplified DNA and AAV vector with restriction enzymes
Ligate the cut DNA sequence into the cut AAV vector
Transform bacteria with the recombinant AAV vector containing your DNA sequence
Purify the AAV vector DNA and verify the cloned DNA sequence is correct by Sanger sequencing
Transfect the AAV vector into human cells using a “helper plasmid” that contains replication and capsid genes → collect the viruses for injection into a mouse model or human patient
CRISPR-Cas9 or siRNA can also be cloned into the AAV vector, allowing for gene therapy to edit or silence dominant alleles
New viruses cannot be assembled when cells are infected with AAV-gene therapy viruses — the AAV vectors essentially stay “dormant” (the cells must be injected with helper plasmids that contain replication and capsid genes to initiate the replication of AAV vectors!)

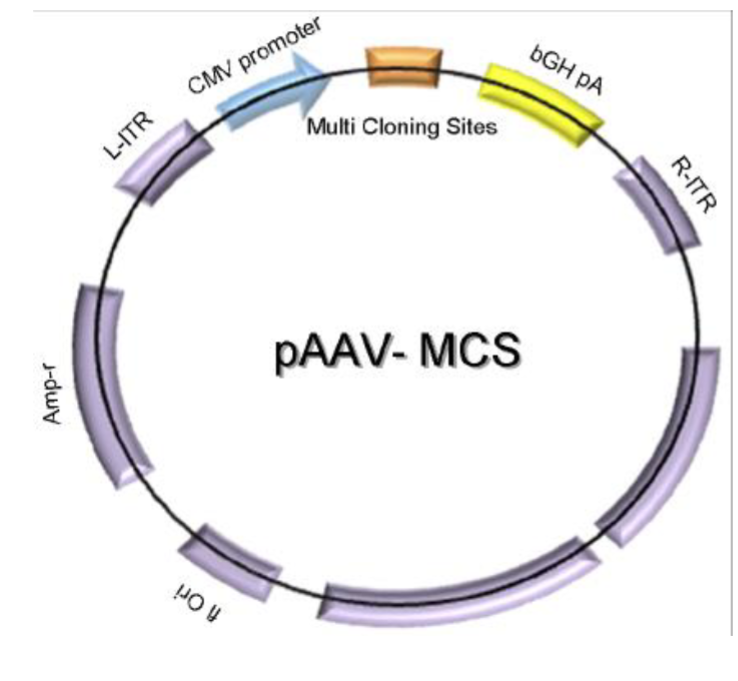
What are some key features of the AAV vector?
AAV vector contains:
L-ITR and R-ITR — left and right inverted terminal repeats
CMV — a strong promoter
MCS — a sequence containing multiple restriction enzyme recognition sites
bGH pA — polyA adenylation site
AmpR — antibiotic resistance gene for selection in bacteria
Ori — origin of replication in bacteria
Transfection
The uptake of foreign DNA by plant and animal cells (eukaryotes) through artificially introducing DNA or RNA
Transformation
The uptake of foreign DNA by bacteria, yeast, and plant cells (prokaryotes) → can happen naturally or be induced artificially for genetic engineering
Compare transformation and transfection.
Transformation is for prokaryotes (and can occur naturally as well as artificially), while transfection is for eukaryotes (can only occur artificially)
Leber congenital amaurosis (LCA)
A rare type of inherited eye disorder that causes severe vision loss at birth (the most common cause of inherited blindness in childhood)
Found in 2-3 out of 100,000 babies
Rod cells in the retina become damaged and die
Coronaviruses (CoVs)
A highly diverse family of enveloped single-stranded RNA viruses that infect humans, mammals, avian species, livestock, and companion animals
If AAV vectors cannot replicate by themselves, what are the dangers of introducing them into patients?
Patients could have an overactive and potentially deadly immune response to the viral load
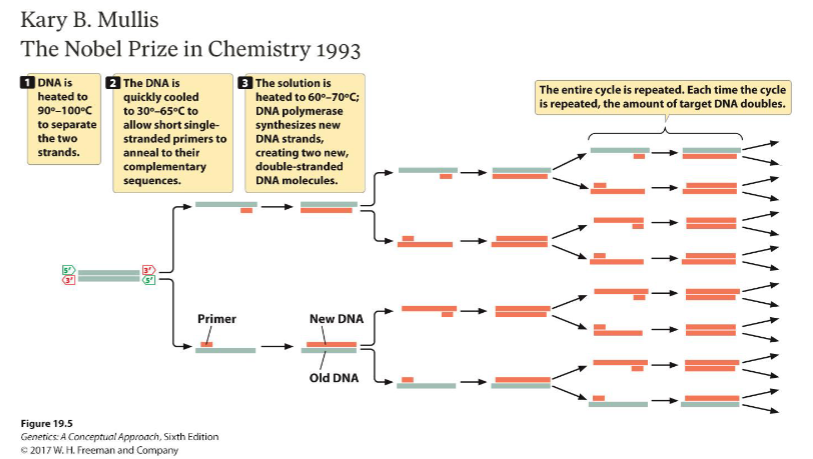
Describe how Kary Mullis’ 1984 invention of Polymerase Chain Reaction (PCR) allowed researchers to synthesize mRNA.
PCR is a method to amplify DNA
Heat DNA to 90-100°C to separate strands
Cool DNA to 30-65°C to allow short single-stranded RNA primers to anneal
Heat DNA to 60-70°C to allow DNA polymerase to synthesize new DNA
Repeat for as many cycles as needed — each time the cycle is repeated, the amount of target DNA doubles (increases exponentially)
PCR can be modified to synthesize mRNA — use RNA polymerase instead of DNA polymerase!
Describe Dr. Drew Weissman’s and Dr. Katalin Kariko’s discovery of how mRNA can be expressed in mammalian cells without triggering the innate immune system response.
Kariko was trying to inject mRNA into mammalian cells, but she couldn’t understand why her experiments weren’t working
Weissman suggested that the reason why mRNA was being destroyed by the cells was because mRNA activated the cells’ innate immune response
Toll-like receptors detect microbial products in mammals and activate the innate immune response → toll-like receptor 7 (TLR7) recognized ssRNA
Had to find a way to modify ssRNA so that it wouldn’t activate TLR7 → which nucleotide in ssRNA activates the innate immune response?
Uracil in foreign ssRNA activated the response — uracil in innate ssRNA doesn’t activate the response because it is modified as pseudouridine
Would gene therapy work better for a recessive disease or a dominant disease?
Gene therapy only works for recessive diseases, because if you are supplying a wild-type form of the gene, then the recessive allele can be suppressed by the therapeutic allele, but a dominant allele will still have a dominant effect and cannot be suppressed by the therapeutic allele
What is one of the major challenges with viral gene therapy?
Accurate and targeted delivery of the AAV vectors to a specific organ or tissue in the cell
A large gene of interest that is too big for the AAV vector to package