Bonding + Crystals
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
What are the different types of bonding forces?
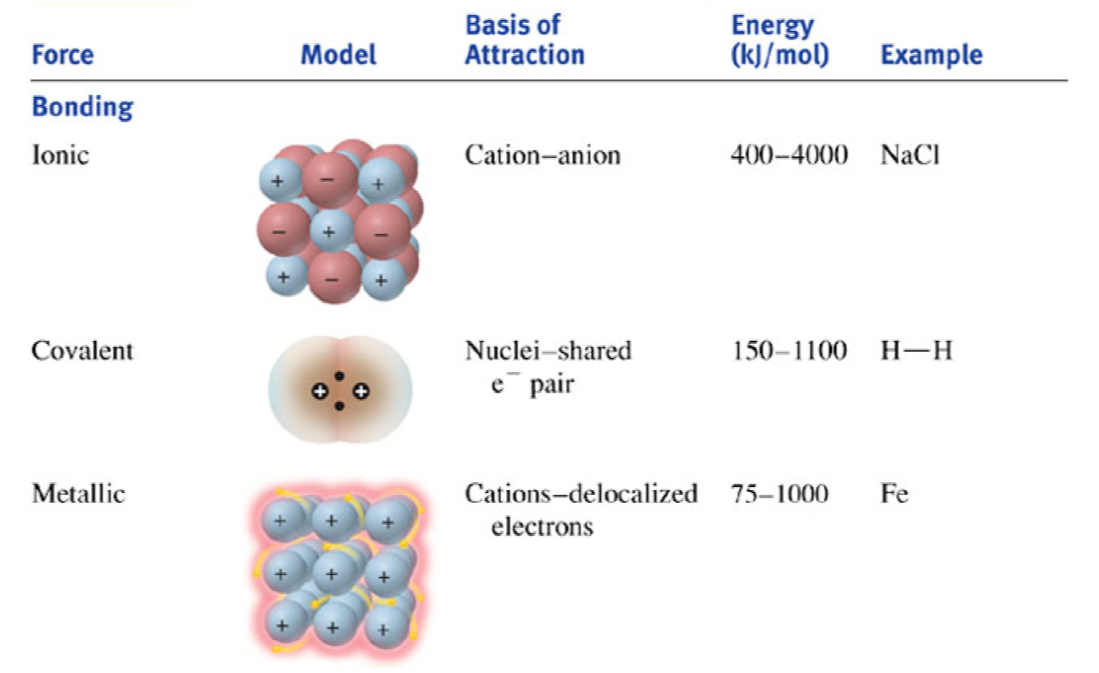
What are the different types of non-bonding (intermolecular) forces?
ion–dipole
attraction between an ion (positive or negative) and the partial charge of a polar molecule
strength: very strong (second only to covalent/ionic bonds)
H-bond
strong type of dipole–dipole interaction where H is covalently bonded to a very EN atom (N, O, or F) and interacts with another EN atom nearby
strength: stronger than regular dipole–dipole, weaker than ion–dipole
dipole–dipole
attraction between the positive end of one polar molecule and the negative end of another polar molecule
strength: moderate
4. ion–induced dipole
an ion distorts the electron cloud of a nearby nonpolar molecule ➡ temporary dipole that it then attracts
strength: Weaker than ion–dipole, depends on how easily the nonpolar molecule can be polarized
5. dipole–induced dipole
polar molecule distorts the electron cloud of a nonpolar molecule ➡ temporary dipole
strength: Weaker than dipole–dipole
6. dispersion forces (London Forces, van der Waals)
attraction caused by temporary fluctuations in electron distribution that create instantaneous dipoles, which then induce dipoles in neighbouring molecules
What are some concepts of atomic solids?
various subtypes
metallic, network, group 18 (noble gases)
wide range of mp
wide range of hardness
insulators/conductors
Does a low m.p. mean a weak covalent bond?
No, it’s the intermolecular forces that are broken.
What are some characteristics of ionic solids?
characterized by cationic + anionic species associated through electrostatic interactions
ionic salts have crystalline structures
high m.p. ➡ strong electrostatic attractions between counterions
insulators when solid (brittle, typically soluble in polar solvents)
What are some characteristics of covalent solids?
strong, directional covalent bonds between their constituent atoms (ex. localized sharing of e-)
high m.p. + bulk hardness
arrangement of atoms ➡ variety of physical properties
only this can lead to variety of physical properties
How do metals form bonds?
pooling their valence e- ➡ “sea” of e- that flows between + around each metal core, attracting them together
metallic bonding e- are delocalized: moves freely throughout metals
What are properties of metals?
malleable: can be pounded into thin sheets
ductile: can form a wire
conduct: electricity + heat
strong bonds that are non-directional ➡ hard to separate atoms but easy to move them
How do intramolecular + intermolecular forces influence the properties of a material?
intramolecular: influence conductivity, thermal expansion + elasticity
intermolecular: govern phase transitions, solubility + vapour pressure
What are the trends in m.p., thermal conductivity + electrical conductivity for molecular, ionic, metallic + covalent network solids?
melting point:
highest in covalent network (very strong bonds)
then ionic (strong attractions)
then metallic (bond strength varies)
lowest in molecular (weak forces)
thermal conductivity:
highest in metals (free e⁻ move heat easily)
then covalent networks (heat moves through lattice vibrations)
lowest in ionic + molecular solids (ions fixed or only vibrate)
electrical conductivity:
highest in metals (free e⁻ flow)
ionic solids conduct only when molten or dissolved
molecular + covalent network solids don’t conduct (no mobile charges)
What are the periodic table trends for atomic radius, e- affinity, EN, ionization energy, metallic character + nonmetallic character?
atomic radius:
across period: ➡ decreases (more protons ➡ stronger nuclear pull ➡ smaller radius)
down group: increases (more e- shells ➡ larger radius)
e- affinity:
across period: increases (atoms more readily gain e-)
down group: decreases (valence e- further from nucleus ➡ weaker attraction)
EN:
across period: increases (atoms more strongly attract e-)
down group: decreases (valence e- further from nucleus ➡ weaker attraction
ionization energy:
across period: increases (stronger nuclear charge ➡ harder to remove an e-)
down group: decreases (valence e- further from nucleus ➡ easier to remove
metallic character:
across period: decreases (elements less likely to lose e-)
down group: increases (elements more likely to lose e-)
non-metallic character:
across period: increases (elements more likely to gain e-)
down group: decreases (elements less likely to gain e-)
What are the differences between crystalline + amorphous solids, and what factors influence their formation?
crystalline:
ordered arrangements with long-range repeating units (periodicity)
made when constituent atoms, ions, or molecules can organize into regular lattices
crystalline state is more thermodynamically favourable
amorphous:
no long-range order; only short/medium-range order over a few Å
lack long-range translational order (no periodicity)
most solids from chemical reactions are amorphous unless annealed (requires time for atoms to rearrange)
formation favoured in kinetic-based processes, even though less thermodynamically stable
What’s a common misconception about solids?
That all solids crystalline, but many are amorphous initially.
What are super-cooled liquids?
Materials that may never crystallize.
What is unit cell?
Smallest portion of the crystal that when stacked together repeatedly can reproduce the entire crystal.
What is a lattice + basis?
lattice: imaginary 3D pattern of points
each lattice point corresponds to a position of highest probability for finding an atom/ion
basis: specific arrangement of atoms associated with each lattice point, characteristic of the mineral.
lattice + basis = crystal structure
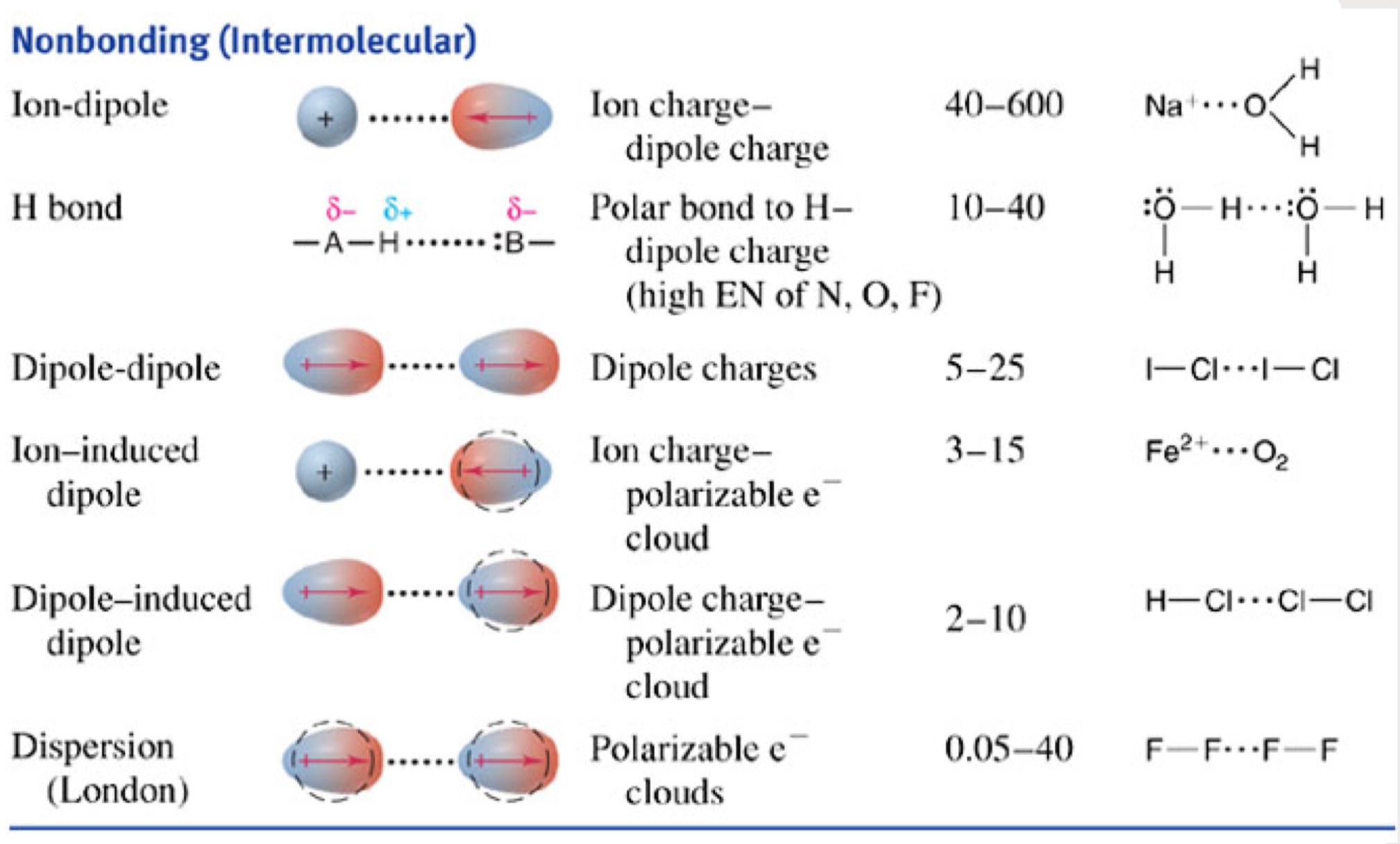
What are the differences between cubic, tetragonal + orthorhombic unit cell lattice types?
cubic
all sides equal (a = b = c)
all angles 90°
can be simple, body + face-centered
tetragonal
2 sides equal (a = b ≠ c)
all angles 90°
simple or body-centered
orthorhombic
all sides unequal (a ≠ b ≠ c)
all angles still 90°
simple, body, end or face-centered
What’s the difference between simple, body-centered + face-centered cubic?
simple: lattice points only at the corners of unit cell
body-centered: lattice points at corners + one additional point in center of unit cell
ball in box
face-centered: lattice points at corners + center of each face of the cell
nothing in box; vacant in middle
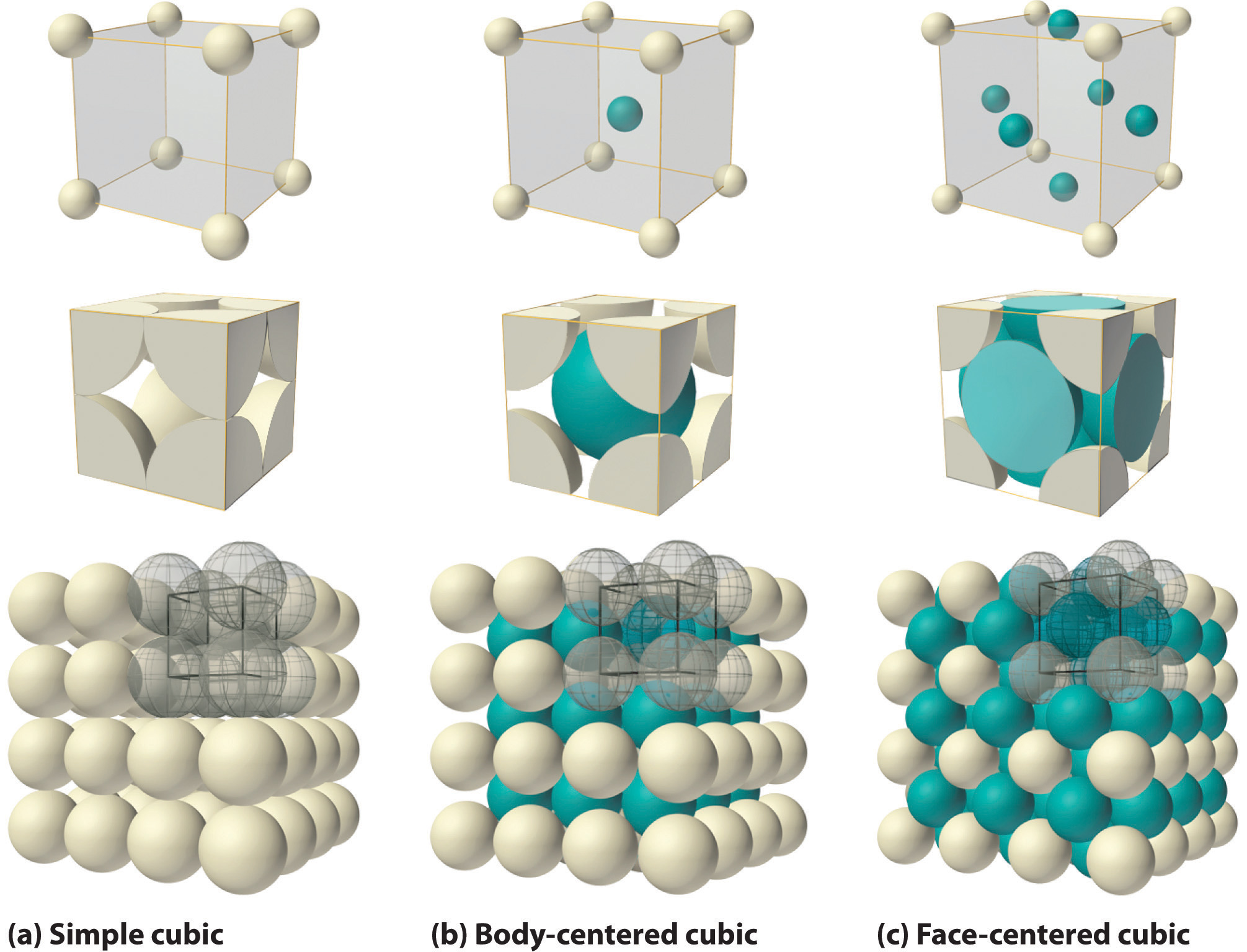
If atoms are rigid + non-interacting, what is the most efficient packing of spheres in 2D?
A closest-packed layer.
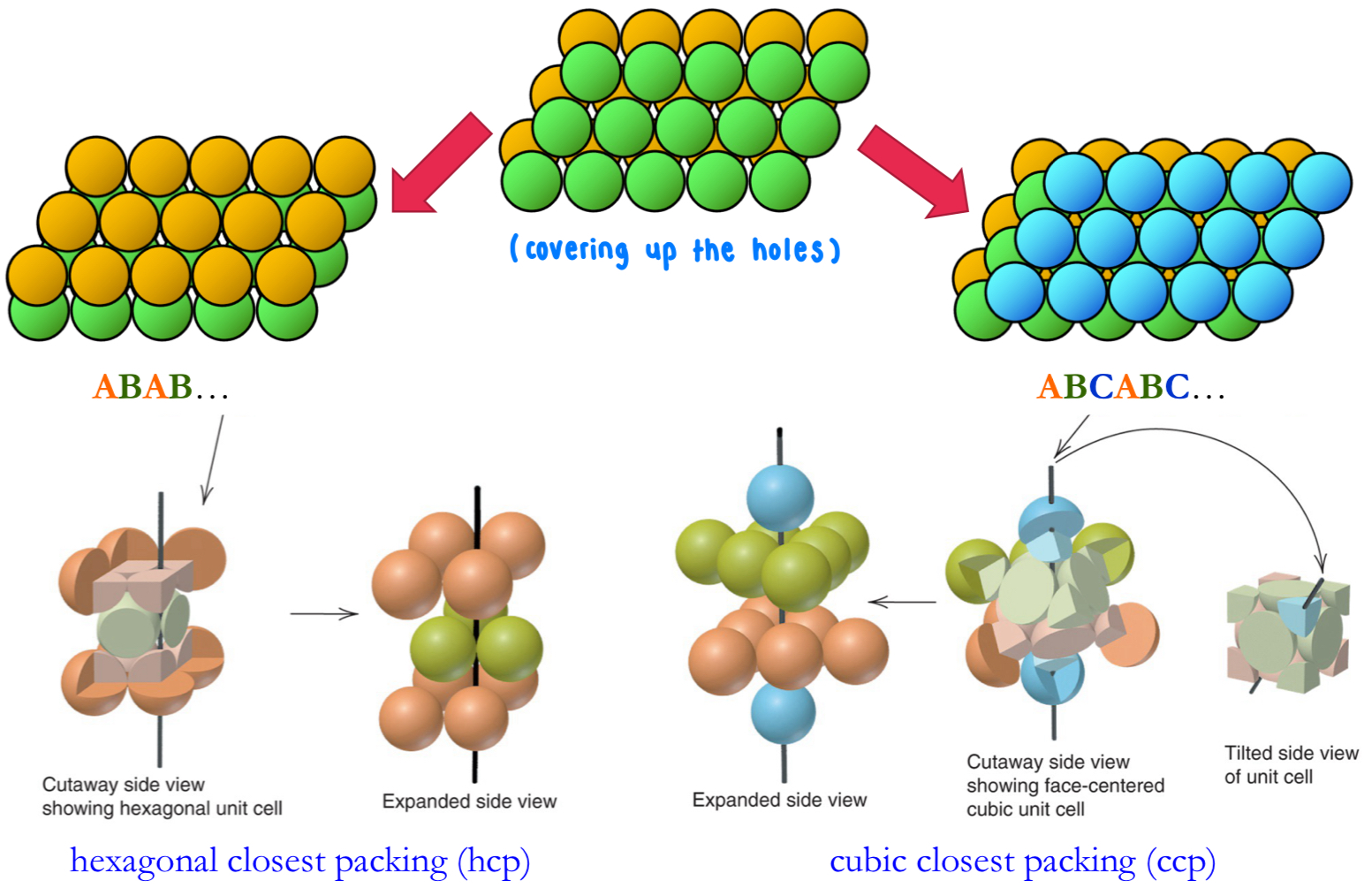
What’s the difference between hexagonal closest packing (hcp) + cubic closest packing (ccp)?
both: types of closest packing in 3D, have CN of 12 + packing efficiency of 74%
hcp:
layer stacking sequence: ABAB…
makes a hexagonal unit cell
ccp:
layer stacking sequence: ABCABC…
makes a cubic unit cell (same as FCC)
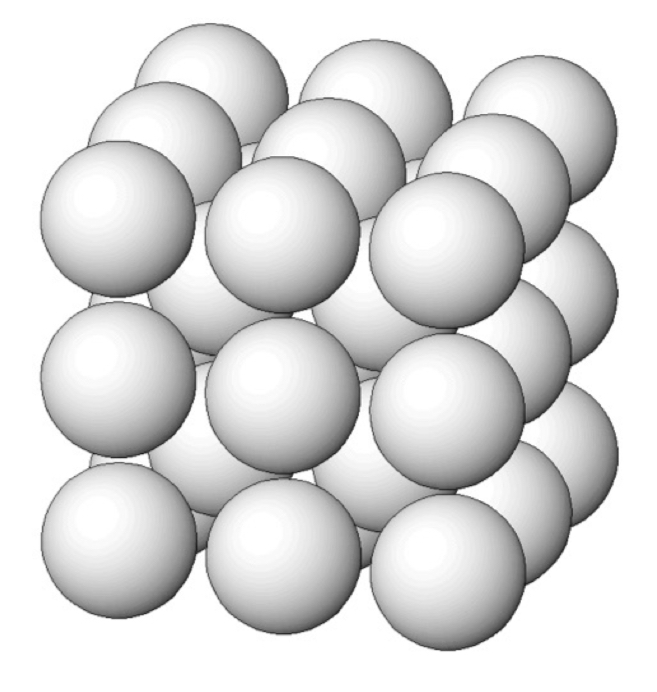
What’s a body-centered cubic (bcc) structure?
atoms are arranged with:
8 atoms at the cube corners
1 atom at the very center of the cube (body)
CN: 8
packing efficiency: 68% (less dense than hcp/ccp, but denser than simple cubic with 52%)
What are alloys + why are they used?
combinations of 2 or more metals that often display improved physical properties compared to pure metals
greater strength, hardness + resistance to corrosion
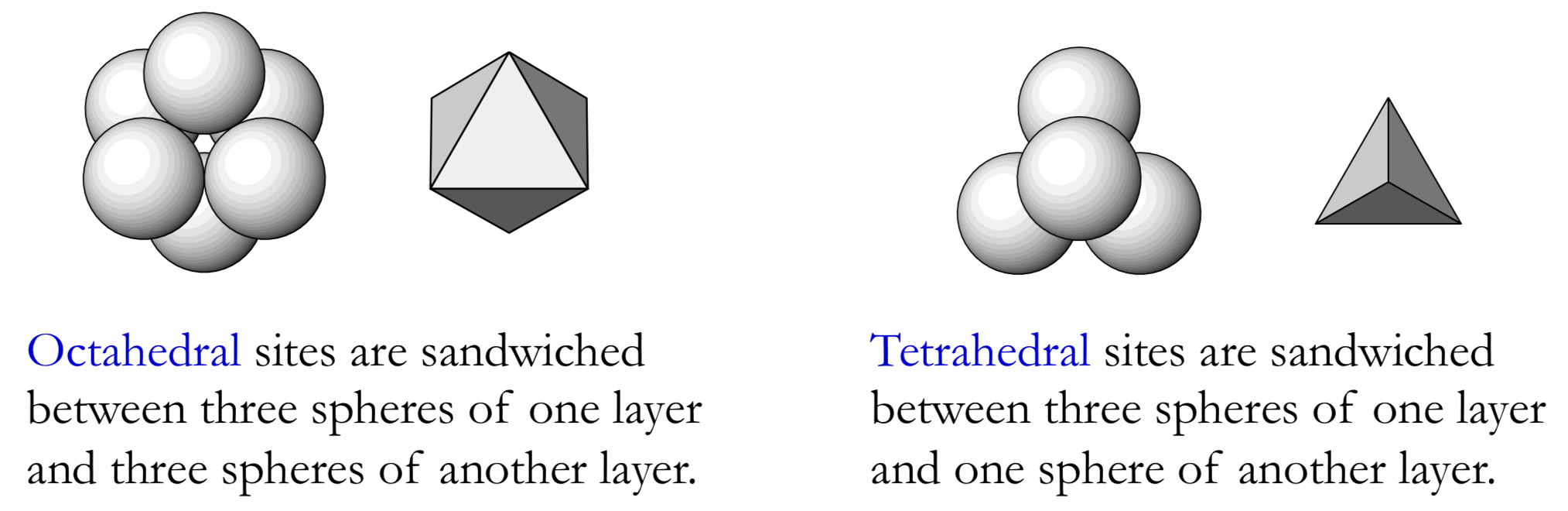
How are ionic solids structured, and what are octahedral + tetrahedral sites?
ionic bonding: non-directional electrostatic forces between cations + anions
arrangement:
larger anions form close-packed arrangements
smaller cations occupy the holes (interstices) in these arrangements
types of interstices:
octahedral sites: cation is surrounded by 6 anions, sandwiched between 3 spheres from one layer + 3 from another
tetrahedral sites: cation is surrounded by 4 anions, sandwiched between 3 spheres from one layer + 1 from another
What are the general trends for cationic radii for given species + charge?
for a given species + charge:
radius increases as CN increases
radius is larger for high-spin (e- spread out into higher orbitals instead of pairing up) ions than for low-spin (e- pair up in lower orbitals instead of spreading out) ions
for a given charge: radius decreases with increasing Zeff (net (+) charge experienced by e-)
for a given species: radius decreases with increasing ionic charge
Are deviations from the general trends for cationic radii common in many crystals?
Yes due to covalent bonding character as bonding is rarely purely ionic (especially for inorganic species).
Why can impurities (dopants) be added deliberately to a solid?
To improve its electrical, magnetic or optical properties.
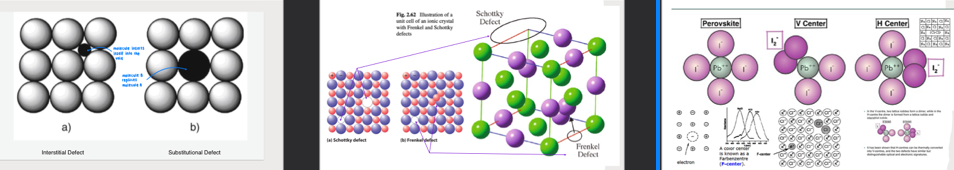
What are point defects?
imperfection for crystalline solids
types:
interstitial: extra atoms that occupy the spaces (interstices) between the regular lattice atoms
substitutional: foreign atoms that replace a host atom in lattice
voids: missing atoms in lattice where a host atom should be
Schottky: paired set of vacancies (one cation + one anion vacancy ➡ charge neutrality)
Frenkel: cation leaves its normal site + moves to interstitial site ➡ vacancy-interstitial pair
F-centre: anion vacancy that traps an e-
H-centre: extra anion that occupies an interstitial site in lattice
What are linear defects?
imperfection for crystalline solids
types:
edge dislocations: book with pages slightly pushed in middle- the edge of the extra plane is dislocation line
screw dislocations: lattice spirals around dislocation line
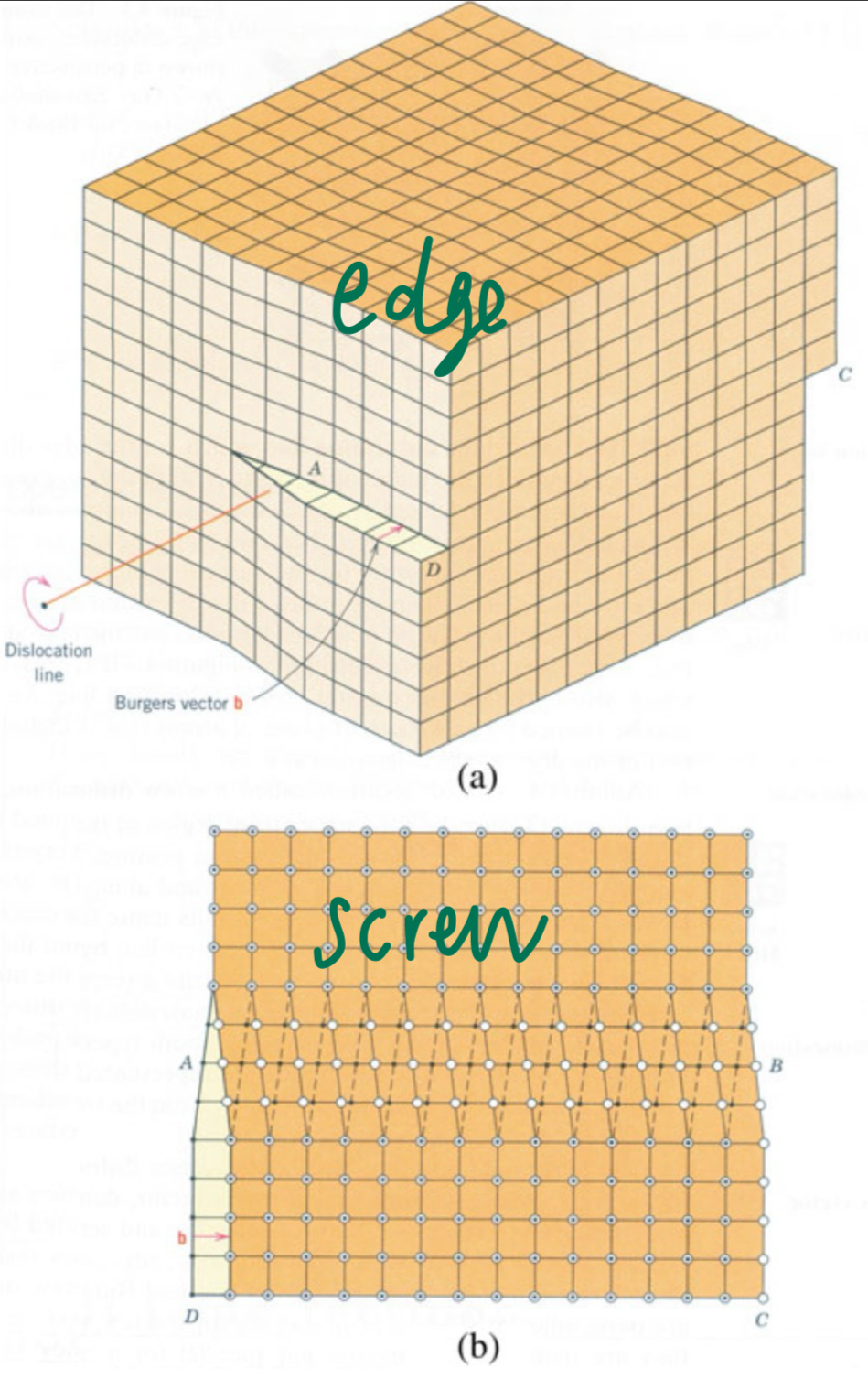
What are surface defects?
imperfection for crystalline solids
types:
grain boundaries: two crystals of same material have different orientations
twin boundaries: type of grain boundary where lattice on one side is mirror image of lattice on other side
surfaces
interfaces
What are volume defects?
Imperfection for crystalline solids where there’s pores, cracks, twins.
What’s the relationship between substitution + doping?
substitution is when a foreign atom replaces a host atom
dopant: foreign atom
difference
substitutional: general replacement (>1%)
doping: case where foreign atoms are at low concentrations (<1%) + randomly distributed, not in every unit cell
What Hume-Rothery rules must be satisfied to form a stable, substitutional solid solution of appreciable solubility?
% difference between solute + solvent atomic radii should be <15%
large mismatches slows diffusion or forces dopant into interstitial sites (for smaller solutes)
matching crystal structures
density of host solvent unit cell must be sufficient to accommodate the solute atoms
similar EN
maximizes solubility + avoids compound formation (e- density would transfer to more EN atoms)
similar valences
maximizes solubility + avoids compound formation
generally: higher-valence solutes dissolve more readily in lower-valence solvents
What ‘s Vegard’s Law?
when you make a solid solution from 2 elements, the size of the crystal’s unit cell (lattice parameter) is roughly the weighted average of the 2 pure elements’ unit cell sizes
key assumptions:
both elements are pure before mixing + have same type of crystal structure
use:
gives quick estimate of lattice parameter without doing detailed measurements
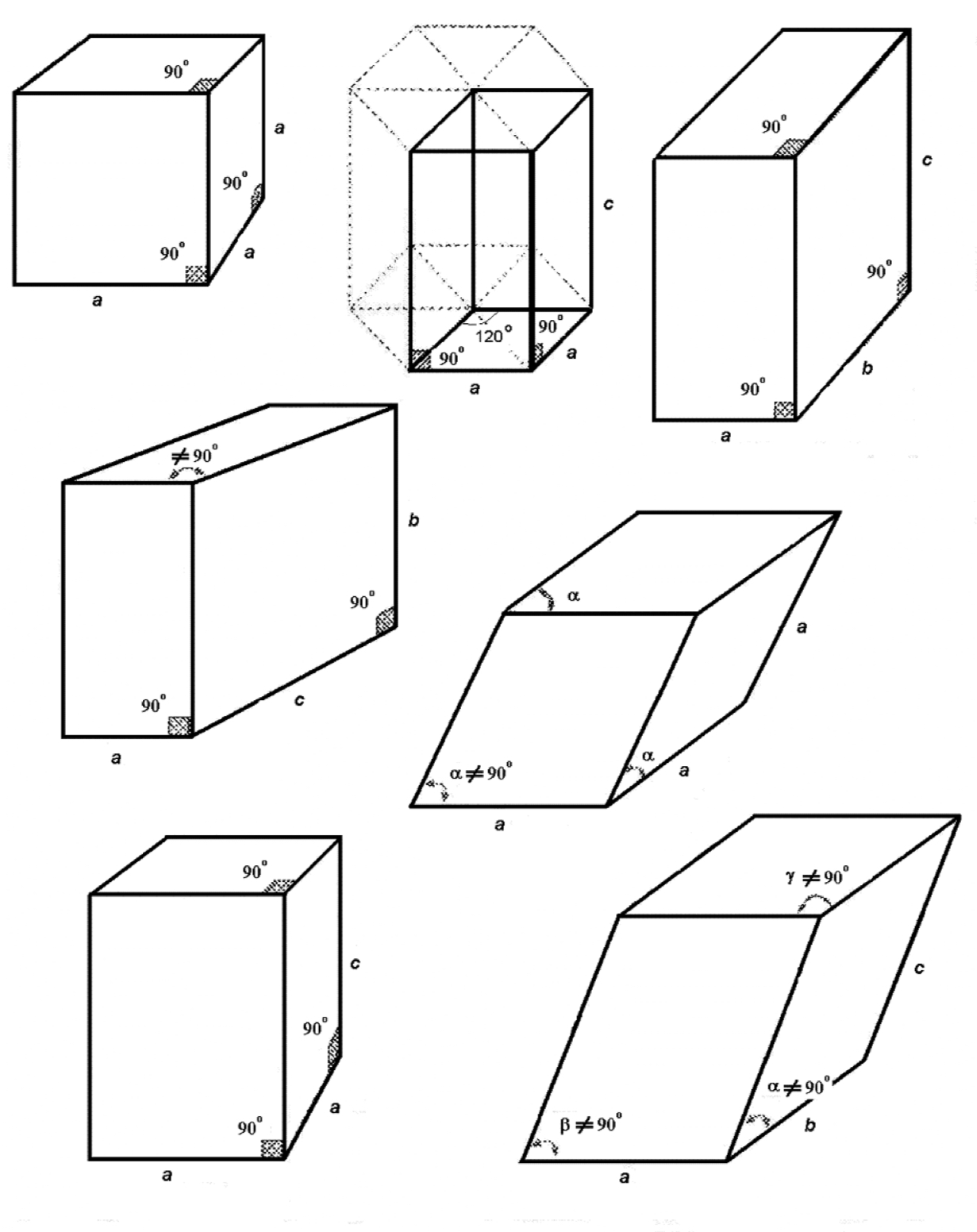
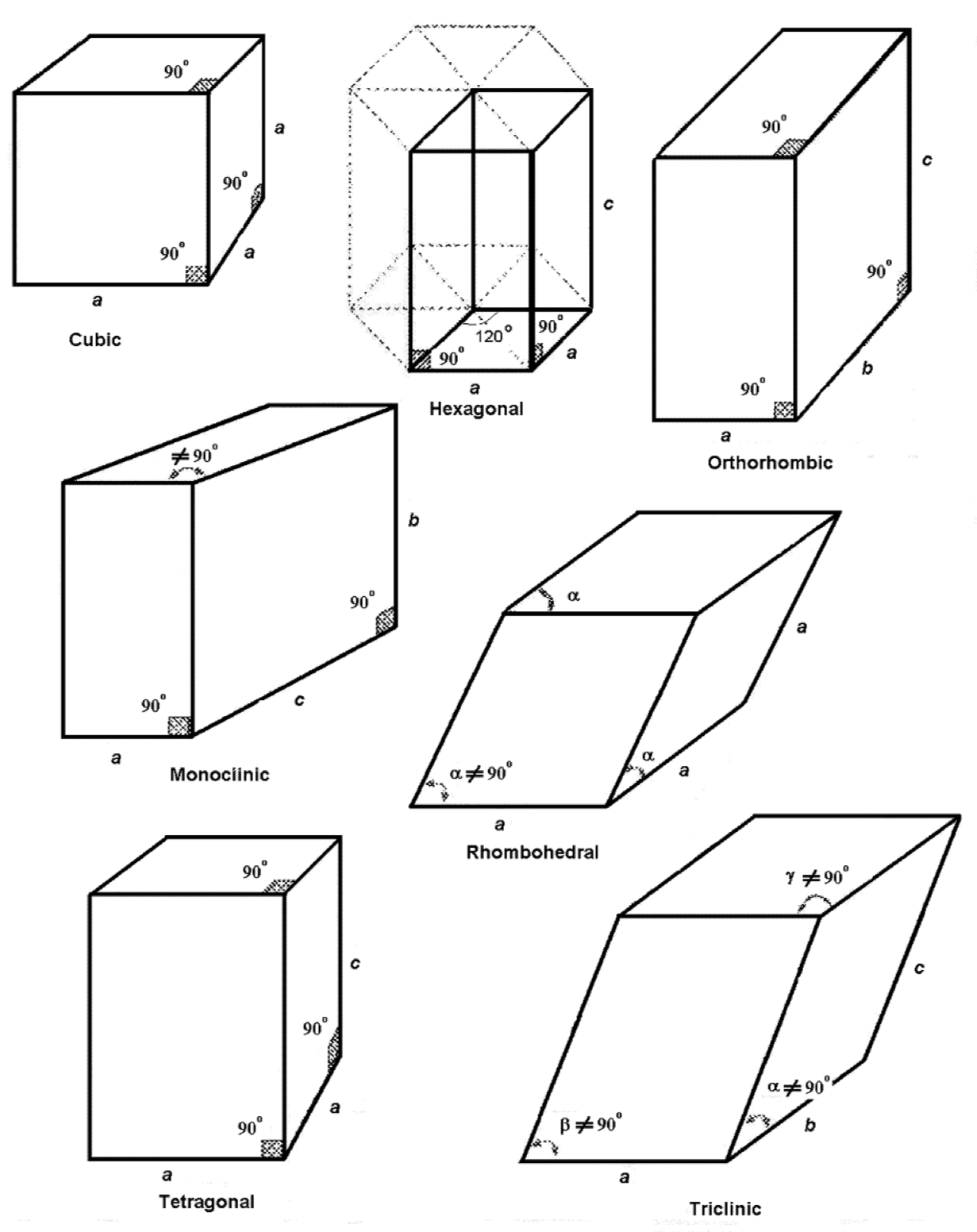
What are the 7 crystal structures?
triclinic
monoclinic
orthorhombic
tetragonal
rhombohedral
hexagonal
cubic
What are some common structure types for 2 ionic solids?
NaCl-type (rocksalt; any alkali metal (group 1)-halogen (group 17) structure)
Cl- ions in ccp arrangement
Na+ ions in all octathedral sites
Li2O-type (antifluorite)
O2- ions in ccp arrangement
Li+ ions in all tetrahedral sites