chapter 17 aromatic compounds
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
criteria for aromaticity
cyclic, planar (SP2 carbons), conjugated pi system, follows huckle’s rule
Huckle’s rule
a compound is aromatic if it fulfills the criteria for aromaticity and contains 4n+2 pi electrons
of the of the four MOs of cylcobutadiene _____ are bonding MOs, ___ are nonbonding MOs, and _____ are antibonding MOs. (hint use a frost circle)
1;2;1
antiaromatic criteria
all criteria(cyclic, planar SP2 carbons, conjugated pi system) for aromaticity except the Hückel rule are met. instead, the compound must have 4n pi electrons
cyclobutadiene has 4 pi electrons so it is ______. this results in _______ which may be relieved by taking a ________ shape
antiaromatic; instability; rectangular
aromatic rings are remarkably _____ so they are usually (unreactive or reactive)
stable; unreactive
(t/f) benzene reacts on its own with Br2
(t/f) cyclooctatetraene reacts on its own with Br2
false
true
larger molecules that fulfill the requirements for antiaromaticity may _______ to avoid being antiaromatic. How does cyclooctatetraene do this?
change shape
it forms a tub shaped structure
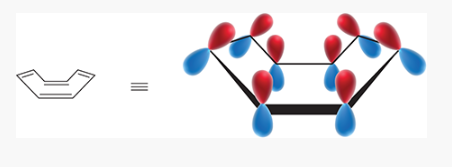
how do you setup a frost circle?
*make sure there are never two degenerate orbitals at the lowest level
annulene
fully conjugated rings
what is the name for benzene as an annulene
[6]Annulene
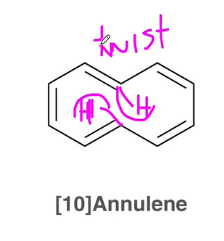
what is special about [10] Annulene
it follows all the rules for aromaticity, however the two inner hydrogens bump each other creating a twist
t/f aromatic rings can contain carbanions or carbocation
true
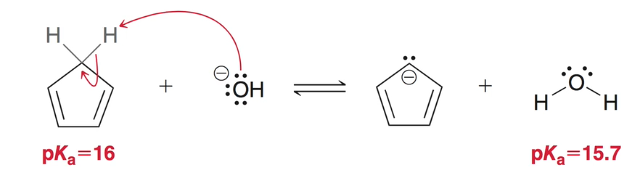
why is cyclopentadiene acidic?
its conjugated base is aromatic
note: while the PKa is slightly higher than water, it is still a much higher PKa than expected from a hydrocarbon
draw the structure of pyridine and pyrrole
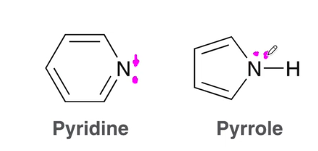
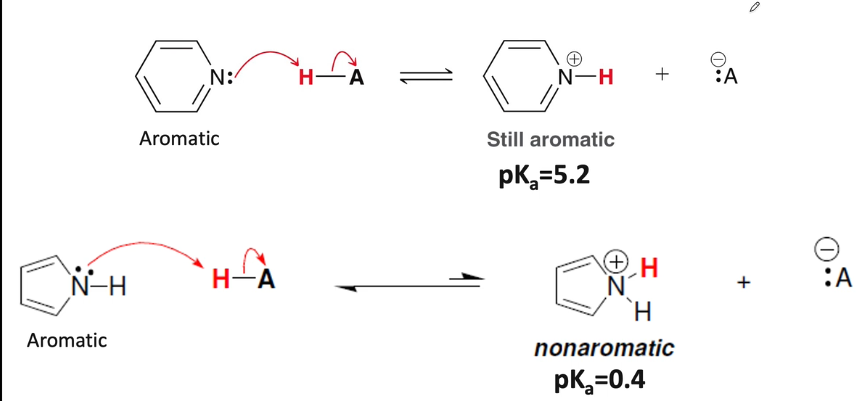
What is the difference between the electronic situations of nitrogen in pyrrole and pyridine?
because of this, which one is a better base?
The electrons on the nitrogen in pyridine are vinylic, localized, and part of the sp2 hybridized orbital. It is not part of the aromatic ring and does not contribute to the Huckel number
The electrons on the nitrogen of pyrrole are allylic, delocalized and part of the p-orbital. they are part of the aromatic ring and do contribute to the Huckel number
Pyridine is the better base as the electrons on the nitrogen may from a bond with H+ without breaking the aromaticity of the molecule
which lone pairs participate in aromaticity?
only the lone pair on the highlighted nitrogen
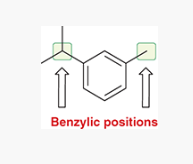
benzylic position
any carbon atom attached directly to a benzene ring
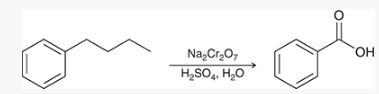
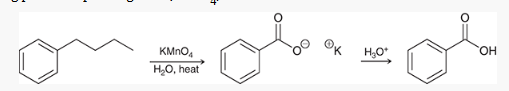
alkyl benzenes may be oxidized at the benzylic position by _______
What does this result in?
chromic acid (formula shown in pic)
results in a benzoic acid
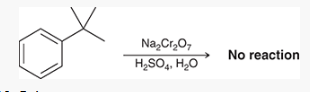
What is the limitations of oxidizing the benzylic position
it may not occur at a quaternary benzylic carbon
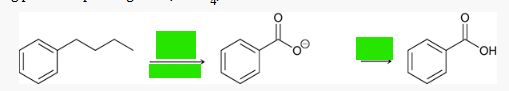
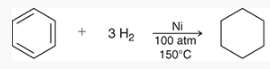
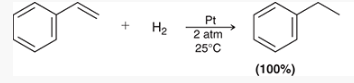
what type of reaction is this? what reagents are used?
free-radical bromination
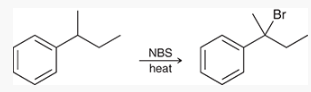
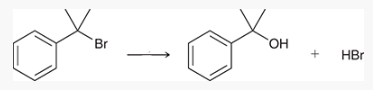
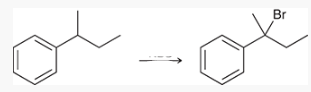
what type of reaction is this? what reagents are used?
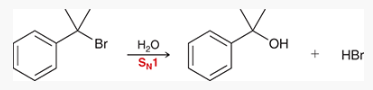
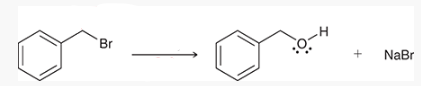
what type of reaction is this? what reagents are used?
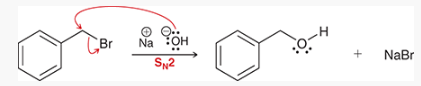
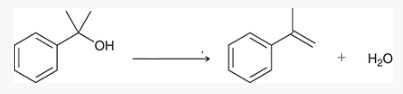
what type of reaction is this? what reagents are used?
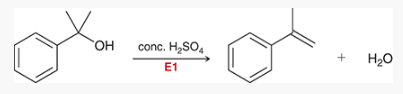
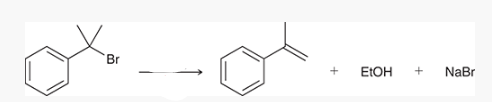
what type of reaction is this? what reagents are used?
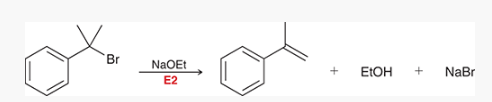
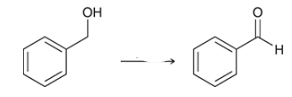
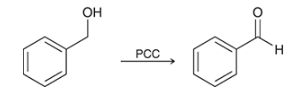
what type of reaction is this? what reagents are used?
oxidation of an alcohol to an aldehyde
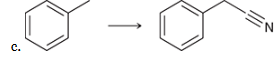
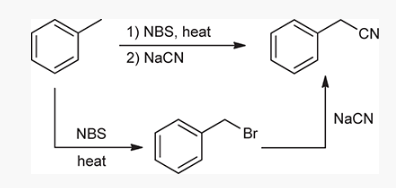
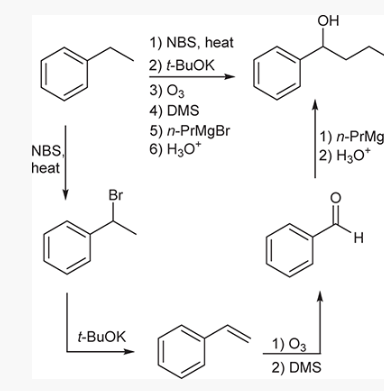
show an efficient synthesis for this reaction
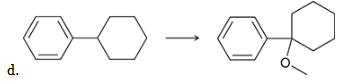
show an efficient synthesis for this reaction
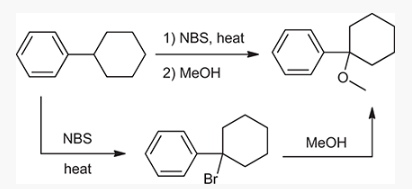

show an efficient synthesis for this reaction
Ozonolysis
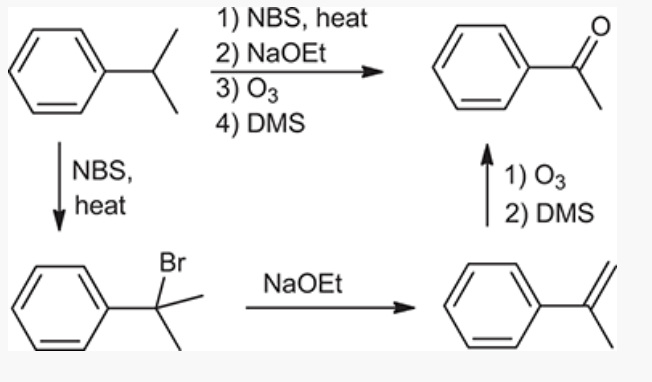
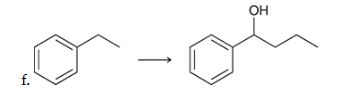
show an efficient synthesis for this reaction


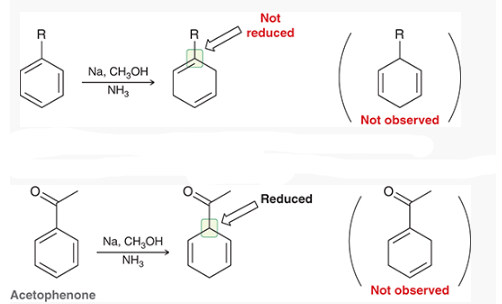
Birch Reduction mechanism (start with benzene)
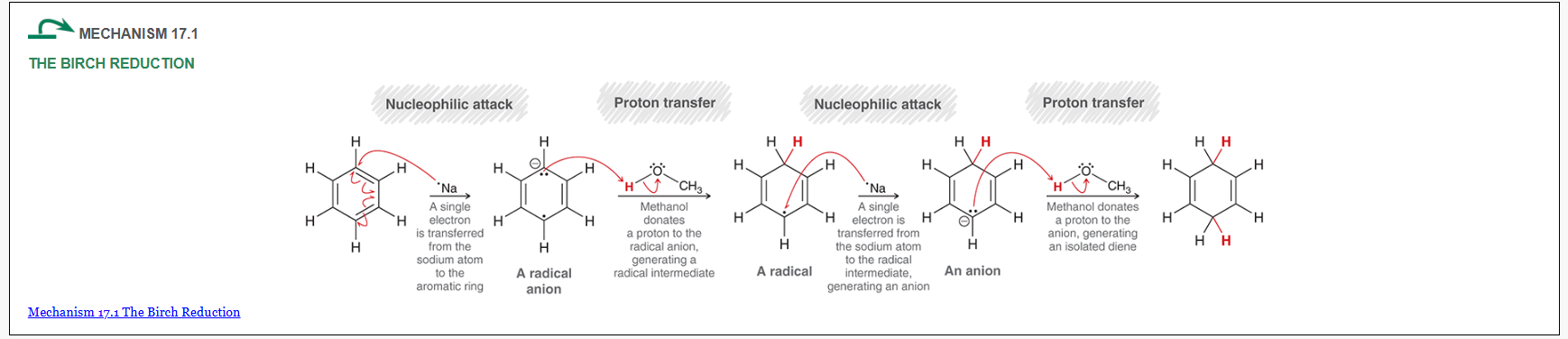

what is this reaction called?
Birch reduction
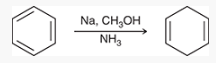
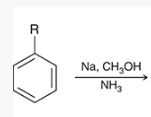
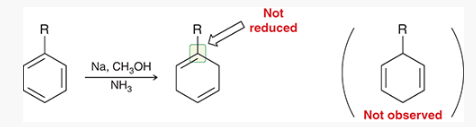
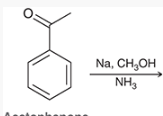
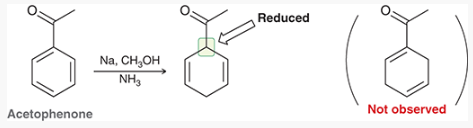
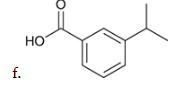
show how and what is made by a birch reduction with this
Na, CH3OH, NH3
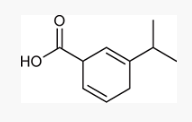
How can you determine the Birch reaction outcome? ie, what are you looking for?
look for any electron withdrawing groups on the aromatic ring. the carbons attached to these groups will be reduced.
If there are electron donating groups, the carbons attached to these groups will not be reduced.