6.1b-6.2/ OZ 2- Wave and Particle Theory of Light, Light and Matter
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
94 Terms
What is electromagnetic radiation?
Energy transmitted as waves
What does the Sun emit and why?
The Sun emits/ radiates a spectrum of electromagnetic radiation because of the nuclear processes going on in its core
What region of the spectrum does the Sun emit radiation in?
Sun mainly gives out visible radiation (light), infrared radiation (heat) and a smaller amount of ultraviolet radiation (wavelengths in the range 270-400nm)
Which region is most of the electromagnetic radiation reaching Earth in?
Mostly visible light and UV radiation
What happens when electromagnetic radiation reaches the Earth's atmosphere and surface?
The Earth’s atmosphere absorbs some of the Sun’s infrared radiation and most of the UV radiation
The Earth’s surface also absorbs radiation from the Sun and is warmed it then re-emits radiation mostly as infrared. The Earth emits much lower frequency radiation than the Sun because it is much cooler
What are the beneficial effects of UV radiation for humans?
Some UV radiation is essential for humans- needed to produce vitamin D in the skin
When the skin is exposed to UV it tans which helps to affect deeper tissue from the effects of radiation
Describe what brief exposure of the skin to the Sun can do?
Brief exposure to the sun can irritate the blood vessels in the skin making it look red and sunburnt
How can the Sun affect the appearance of the skin?
In less extreme cases Sunlight can damage the proteins in the skin so after years of exposure people look wrinkly and leathery
Describe how the Sun can be harmful to humans
Part of this spectrum corresponds with the energy required to break chemical bonds including the chemical bonds in molecules such as DNA
This can cause damage to DNA in the skin cells hit by the Sun’s electromagnetic radiation leading to damage to genes and potential mutations which can lead to skin cancer
How does the frequency of electromagnetic radiation from the Sun affect the scale of damage it causes?
Higher frequencies, more energy, more damaging
What are the effects of visible electromagnetic radiation from the Sun on the skin?
Temporary reddening
What are the effects of UV-A electromagnetic radiation from the Sun on the skin?
Quick tanning which fades quickly, causes the skin to age faster. Can also lead to skin cancer but generally not a deep suntan which fades quickly (exam- just say tanning and UVB causes skin cancer), as UVA is lower frequency so lower energy so less damaging radiation
What are the effects of UV-B/C electromagnetic radiation from the Sun on the skin?
Tanning and burning
UVB causes the skin to age faster
Can damage the DNA in cells and cause skin cancer
Main cause of sunburn
Can damage eyes (leading to cataracts), and damage crops
What effect can UVB/C have that is not on humans?
Can damage crops/ cause mutations in crops
Draw a spectrum to show the effects of different frequencies of UV/ visible light radiation on the skin
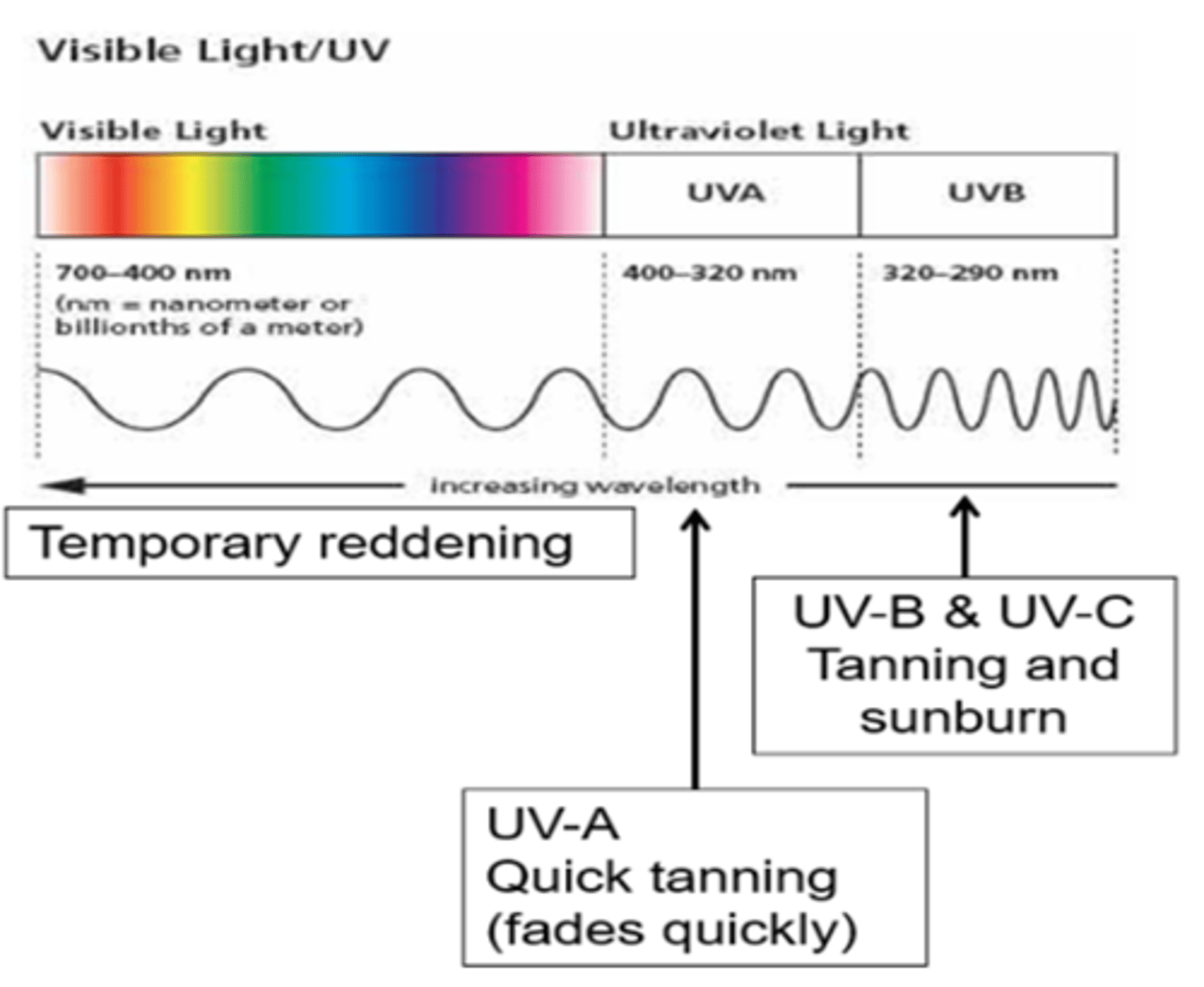
Which part of the spectrum is most damaging?
UV radiation
Describe 4 substances which absorb UV radiation?
CHEMICALS- which absorb much of this radiation. Molecules effective at absorbing UV radiation- oxybenzone, cinoxate
GLASS- the glass in a window lets the visible light through but absorbs the high-energy UV radiation so it never reaches your skin- cannot burn through a window
WATER- does let through some ultraviolet so it is possible to burn under water
ATMOSPHERE- best protection from UV, very good sunscreen
What has been developed to protect the skin against high energy UV radiation?
Chemical sunscreens
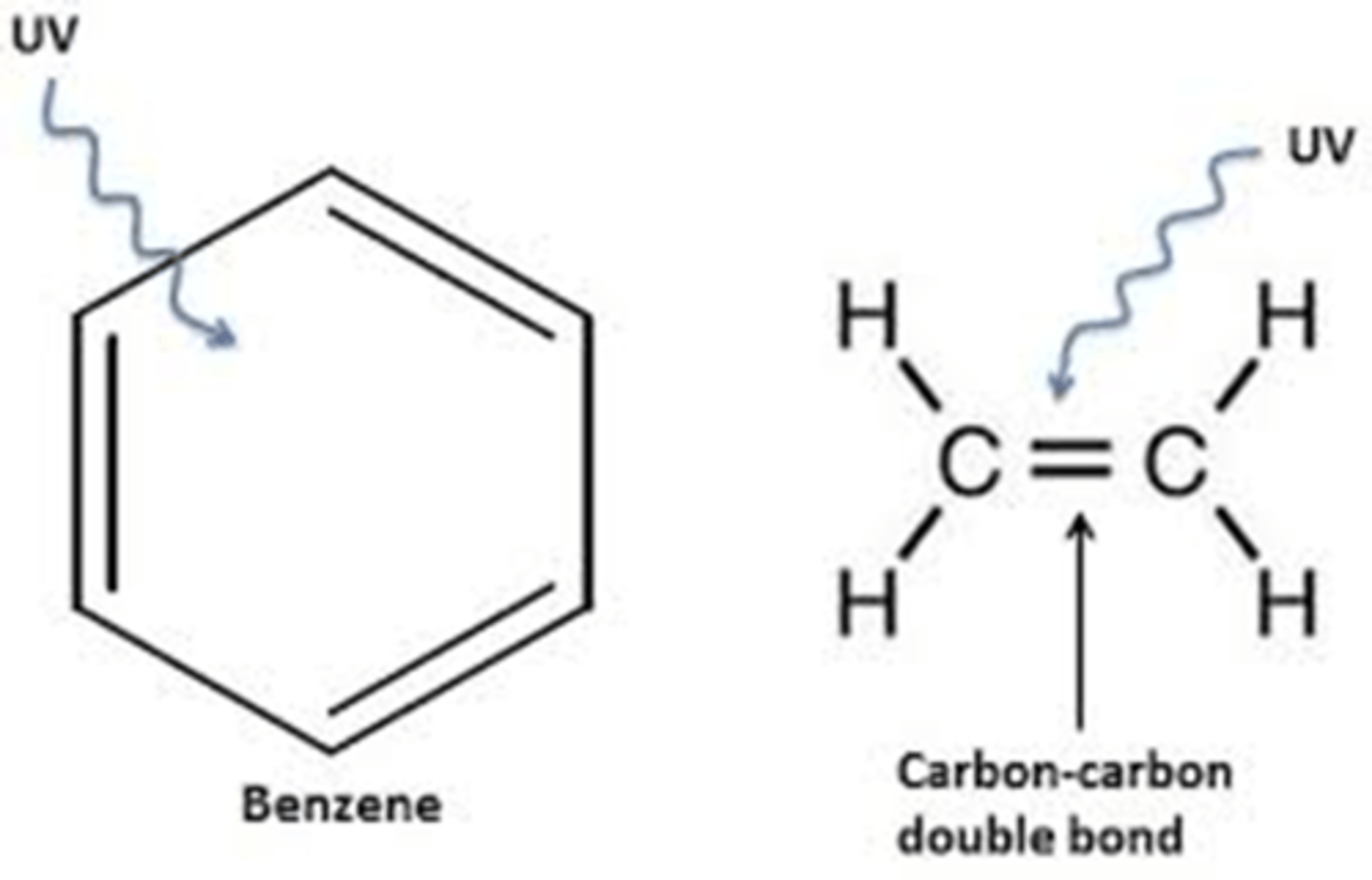
Which chemicals used in sun creams are able to absorb UV radiation and how?
The molecules in sunscreen often contain benzene rings or alternating double and single bonds. (Cannot just be benzene (carcinogens) but can be benzene containing compounds)
When UV light is absorbed the electron in the pi orbitals in these bonds are promoted to higher energy levels
Explain SPF?
SPF is how long it takes the skin covered in Sunscreen to burn (SPF 15- takes 15 x longer to burn than with no sunscreen). SPF only considers burning times, so it only applies to UVB.
Describe an experiment to show the absorbance of UV radiation by suncreams?
Three beakers, different factors of sunscreens on each on cling film, tonic water/ Quinine fluoresce under UV- see how much UV gets through each other
Which atmospheric gas is able to absorb UV radiation?
Ozone
What is the function of the ozone layer?
Protects us from most of the harmful effects of the Sun's ultraviolet radiation/ stops most the harmful UV radiation from reaching Earth
Where does most of the absorption of UV radiation in the atmosphere occur?
In the region of the upper atmosphere- the stratosphere
EXAM TIP- should you say high energy UV radiation which is harmful OR harmful UV radiation
High energy UV radiation which is harmful
What is ozone?
A form of oxygen O3
Is ozone harmful to humans?
Yes it is toxic
Where is ozone found?
In the stratosphere
Describe how ozone absorbs UV radiation and how much it absorbs?
When ozone breaks down it absorbs high energy UV radiation so the ozone layer removes all of the high energy UVC radiation and about 90% of the UVB radiation (all UV with shorter wavelengths (less than 320nm))- these types of radiation are harmful to humans and most other life on Earth
Which parts of the electromagnetic spectrum does ozone absorb?
Ozone absorbs high energy UV radiation in the region 10.1x1014 to 14.0 x 1014 Hz which includes the UV-B and UV-C region
Is ozone good or bad for the environment?
Good and bad for the environment depending on where it is found
Stratosphere- good, protects against UV
Troposphere- bad, involved in photochemical smog
Is there any life in the stratosphere, why?
There is no life in the stratosphere- the high energy UV radiation would break down the delicate molecules of living things
Even simple molecular substances are broken down by the UV radiation in the stratosphere
What can happen when covalent bonds in molecules in the stratosphere break due to the UV radiation?
Some of the covalent bonds break to give fragments of molecules called radicals
What can happen to molecules even higher up than the stratosphere?
Higher up in the atmosphere the radiation is powerful enough to knock electrons out of atoms, molecules and radicals- ions are produced forming the ionosphere
Why is the ozone layer hotter than other parts of the atmosphere?
The atmosphere/ ozone breakdown reaction converts ultraviolet radiation into heat so the ozone layer is hotter than other areas of the atmosphere
How is ozone measure?
Dobson ozone spectrophotometer- used to measure stratospheric ozone from the ground. There are world-wide ozone monitoring stations which still continue today
Exam Question: Why is it important to life on the Earth's surface that the concentration of ozone in the stratosphere is maintained? (1 mark)
It absorbs harmful UV radiation from the Sun.
ALLOW 'keeps out UV' OR 'filters UV'
ALLOW increased UV could cause skin cancer
OR increased UV could cause cataracts
OR increased UV could cause mutation of crops
What happens when ozone is present in the troposphere, what types of chemical does this make it?
Ozone causes photochemical smog so it is a pollutant in the troposphere
How does ozone get into the troposphere and how does this then cause a photochemical smog?
Occurs in the troposphere due to the effect of sunlight on mixtures of nitrogen dioxide and hydrocarbons- occur naturally from some sources but mostly from vehicle engines and power stations
In industrialised areas and cities with many cars the ozone mixes with solid particles of carbon and many other substances to create an air pollutant called photochemical smog
What problem does a photochemical smog cause?
Haziness and reduce visibility, irritation and respiratory problems for many people especially those with asthma, dangerous for animals, can damage plant material
Name one place where photochemical smogs have occurred?
Mexico City
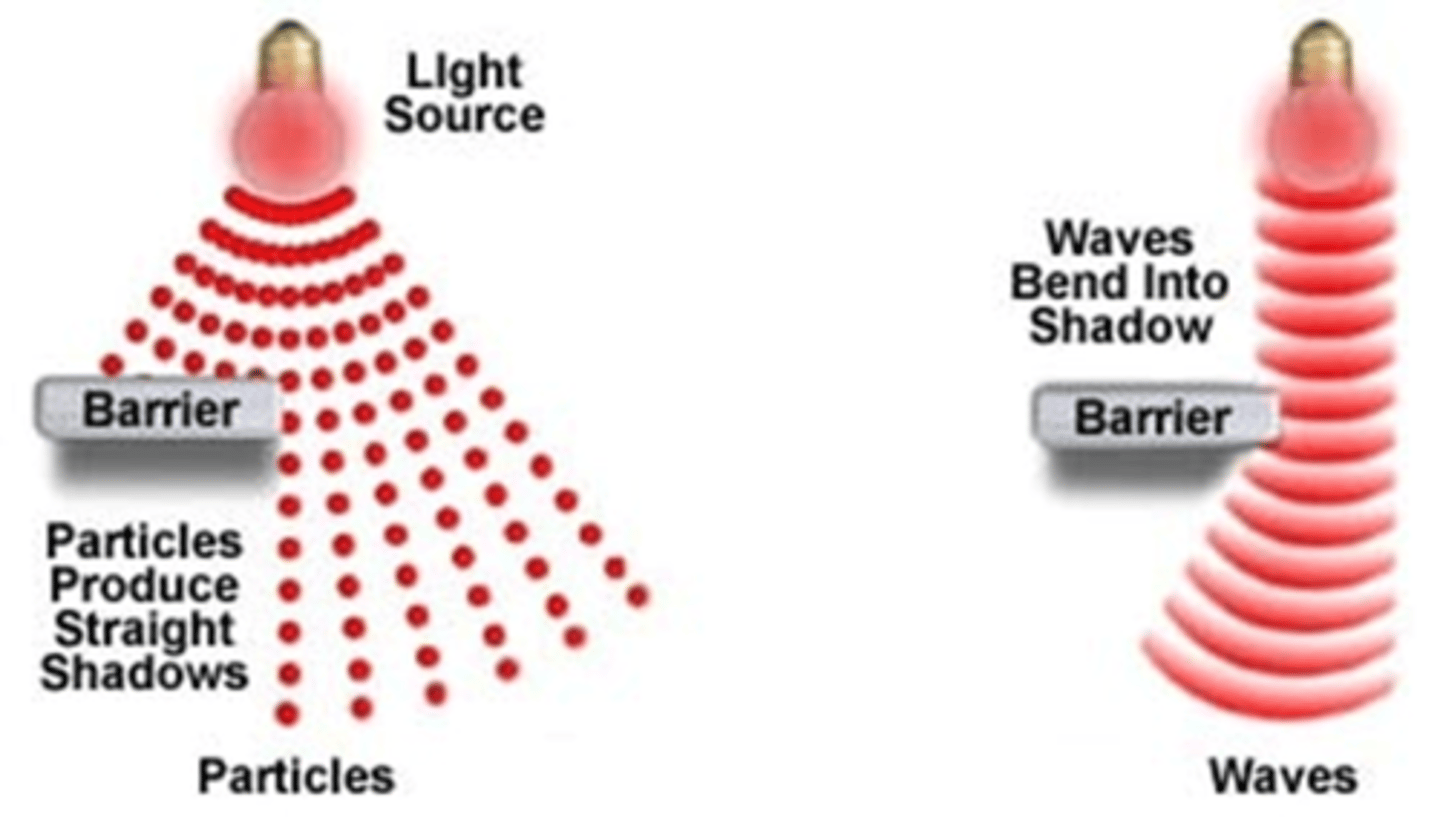
What two theories can be used to describe the behaviour of light?
The behaviour of light can be described using the wave model or the particle model
What does the particle theory of light say?
The behaviour of light can sometimes be explained by thinking of it as particles called photons
Describe what a photon is and how they can be said to be travelling
Tiny packets of energy which travel in a stream which makes up light
What decides the energy of a photon?
The energy of photons is related to the position of light in the electromagnetic spectrum according to the equation E= hv
Example: photons with an energy of 3 x 10-19J correspond to red light
What does the wave theory of light say?
Like all EM radiation- light behaves like a wave, characteristic wavelength and frequency
The speed of light c is the same for all kinds of EM radiation
Does either model fully explain all the properties of light?
No, neither theory fully explains all the properties of light- some properties explained more by one model than the other
Describe what sort of shadow forms with the wave and particle theory of light
What is the symbol and unit for frequency?
"nu", Hz or s-1
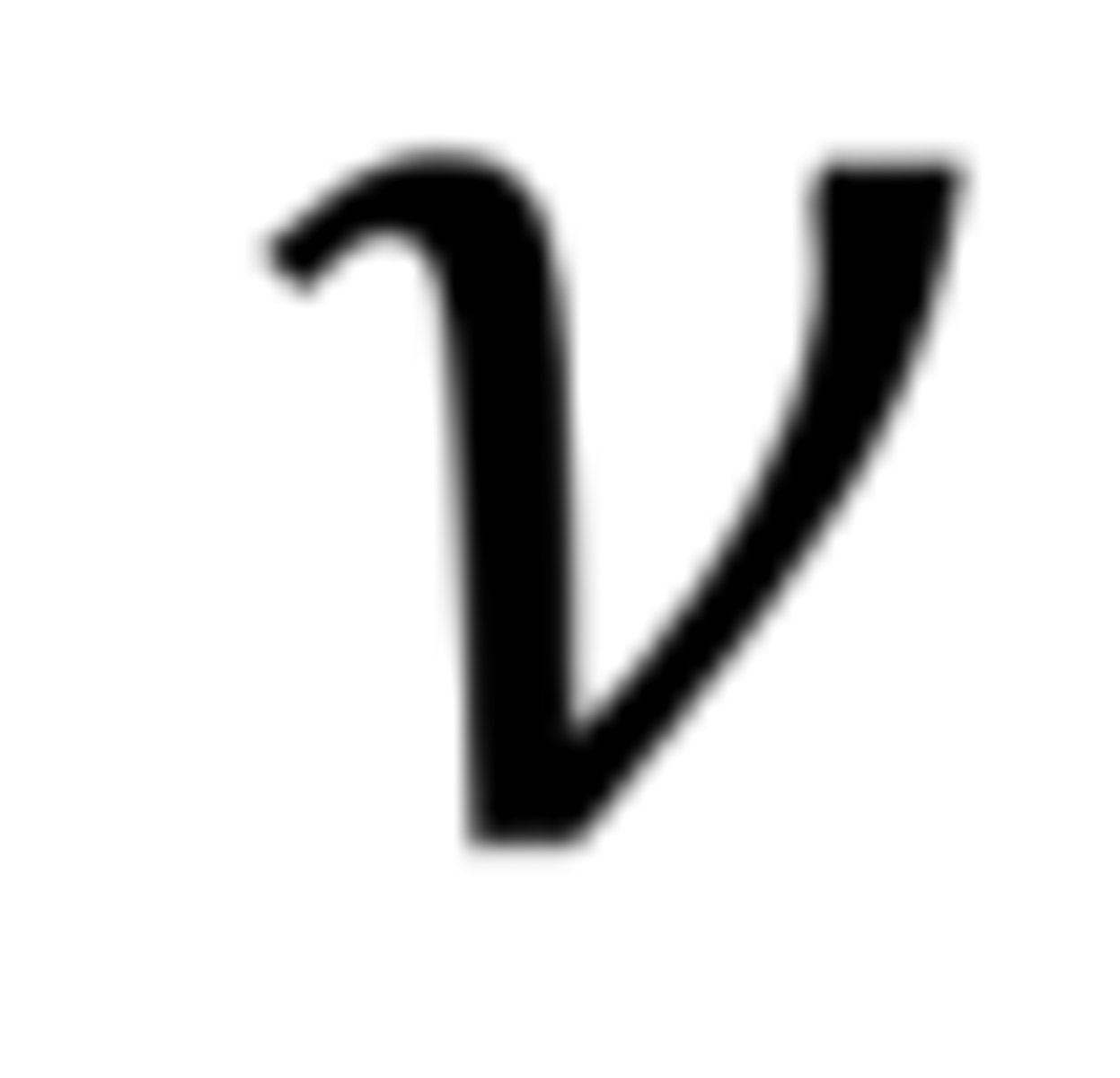
What is the value of c?
The speed of light
c has a value of 3.00 x 108 ms-1 when the light is travelling in a vacuum
Give the equation for the particle theory of light and explain what each symbol means?
E = energy associated with 1 photon in Joules (J)
h = the Planck constant (6.63 x 10-34 J Hz-1)
ν = the frequency of the radiation (Hz)
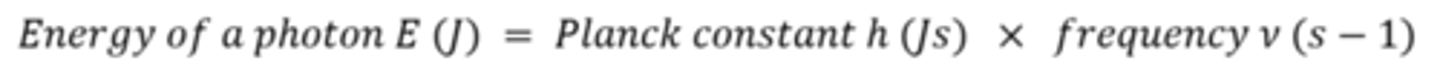
What are the two units for Planck's constant, why are there two units?
J Hz-1 or J s
1 Hz-1 = 1 s
Give the equation for the wave theory of light?
Speed of light = wavelength/ frequency
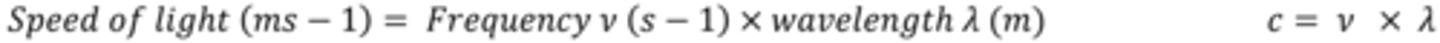
What is the equation for the particle theory of light?
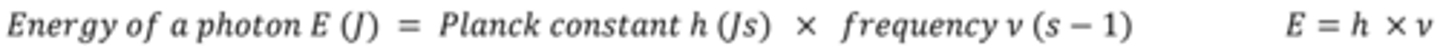
Give the triangle for the particle theory of light equation?
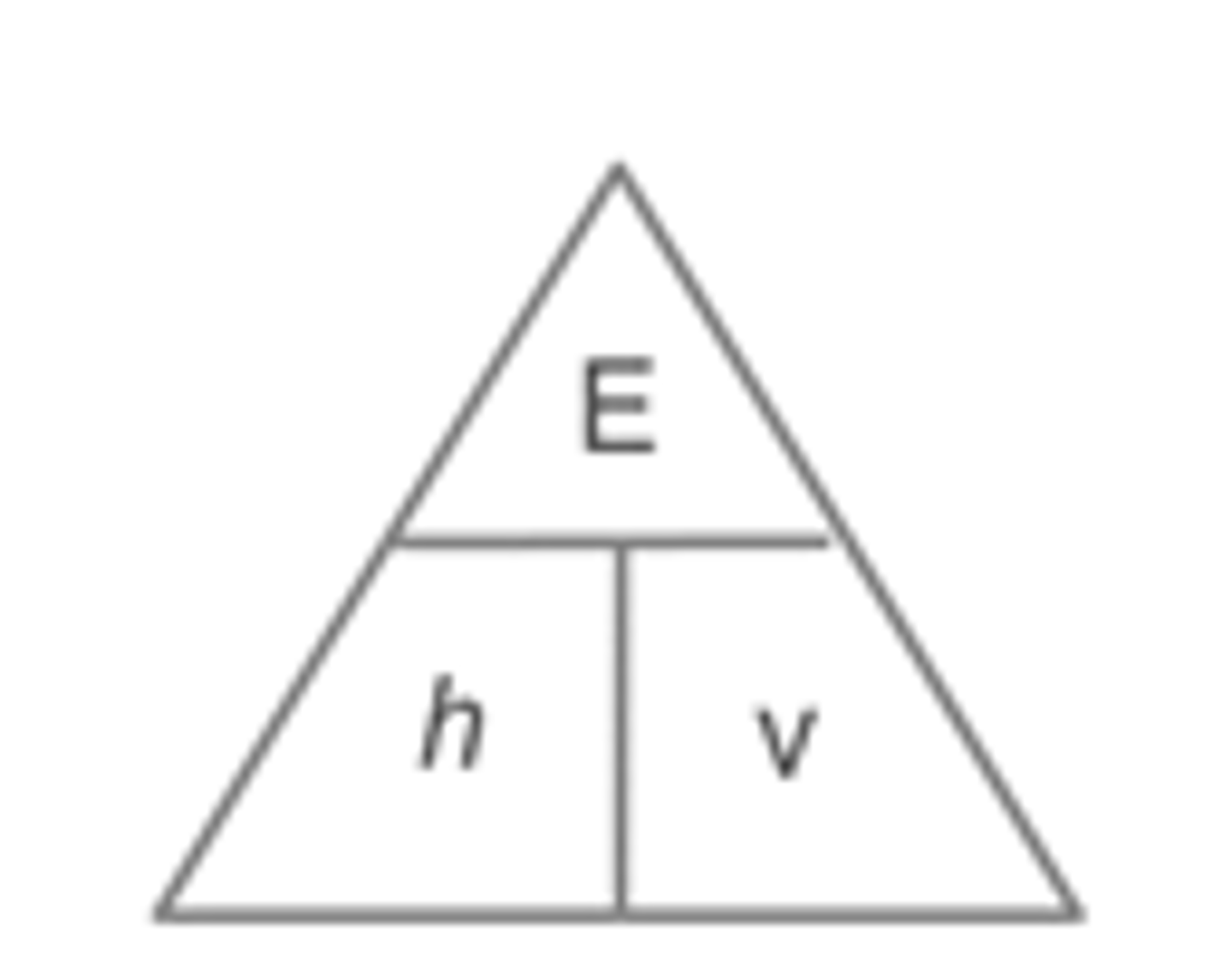
Give the equation which links the wave and particle theories of light?
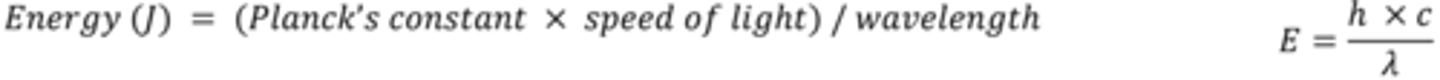
EXAM TIP- If asked in an exam whether a light wave/ other form of radiation will have enough energy to break a bond how should this question be answered?
Use the equations to calculate the energy of the radiation then if it is greater than the bond enthalpy- must state THEREFOR THE e.g. UV radiation will break this bond to get full marks
EXAM TIP- If asked in an exam to determine the bond enthalpy of a bond broken by EM radiation of wavelength x m what should you do?
Calculate the energy of that wavelength then multiply this by Avogadro's constant as bond enthalpy refers to ONE MOLE of bonds of that type.
EXAM TIP- What must you remember to do to the wavelength before any calculations?
Always convert to the correct units- m
1 nm = 1 x 10^-9 m
What is one nm (nanometre) equal to?
1 x 10^-9 m
EXAM TIP- How would you calculate the energy value per mole of photons?
Multiply the value per photon/ for an individual photon by Avogadro's constant (NA)- the number of photons in a mole
What is the effect of electromagnetic radiation on molecules?
Electromagnetic radiation can interact with matter transferring energy to the chemicals involved which can cause changes in the chemicals
What two factors affect the type of energy change in a molecules when it is hit by electromagnetic radiation?
1. The chemicals
2. The amount of energy involved/ transferred/ absorbed corresponding to the frequency of electromagnetic radiation absorbed
What are the four different types of energy change (list as increasing amounts of energy required/ higher frequency radiation)
A molecule has energy associated with several different aspects of its behaviour
· Translation energy (the molecule moving around as a whole)
· Rotation energy (rotation of the molecule as a whole)
· Vibration energy (bonds within the molecule vibrate)
· Electron energy (movement of electrons within the molecule)
Which type of energy change requires the highest energy/ frequency electromagnetic radiation and which required the lowest?
Translational energy- lowest
Then rotations then vibrational
Electronic energy- highest
What is required for the different types of energy change?
Different amounts of energy
How does a different amount of energy correspond to a different type of electromagnetic radiation?
Different types of electromagnetic radiation corresponding to different frequencies have photons of different energy associated with them
How can all energy changes be described in terms of the amount of energy?
ALL types of energy (electronic/translations/ rotational/ vibrational) are all quantised
Electron energy- Why is electron energy quantised?
Electrons can occupy definite/ fixed energy levels so the energy of the electrons is quantised
Electron energy- Which electrons are at the highest energy level?
Outer shell electrons are the highest energy levels so can most easily to higher level
Electron energy- How can electrons change energy levels?
Electrons can jump between energy levels/ to higher energy levels- moving from its ground state to an excited state
Electron energy- Which type of energy of an atom/ molecule changes when the electron moves from one energy level to another?
The electronic energy
Electron Energy- What quantity of energy is needed to excite electrons to higher energy levels, what part of the electromagnetic spectrum does this correspond to?
Energy between 1x10-19 and 1x10-16J corresponding to the visible and UV parts of the spectrum
The amount of energy is fixed/ specific- quantised so only specific frequencies are absorbed.
EXAM TIP- In electron energy changes does the atoms absorb a fixed amount of energy or does the atom absorb a specific frequency?
The atom/ molecule absorbs a fixed amount of energy (quantised) which then corresponds to a specific frequency (Planck's equation- use E= hv to determine frequency of radiation from the fixed amount of energy)
Electron Energy- What happens when UV radiation/ visible light hits a molecule of gas?
The electrons absorb the energy and jump to their next energy level- the energy needed is quantised, so only specific frequencies are absorbed
Electron Energy- Can electron energy changes occur in molecules?
Yes it can happen within molecules- electrons within the molecule now jump to higher energy levels
Electron Energy- How does electron energy relate to emission spectra?
Absorb in visible region- emit in visible region, emission spectra
Vibrational Energy- What happens when the vibrational energy of a molecules changes?
Bonds within the molecule vibrate more/less- kinetic energy
Vibrational Energy- what happens to bonds when they absorb radiation, which equation does this relate to?
They have more kinetic energy (E= hv)
Vibrational Energy- how many joules of energy/ what frequency of radiation is absorbed by molecules/ bonds to cause vibrational energy changes?
1x10-20 to 1x10-19J spacing between vibrational energy levels/ needed found in the infrared radiation part of the electromagnetic spectrum
Vibrational Energy- what does the amount of required energy correspond to?
Spacing between vibrational energy levels
Vibrational Energy- how do vibrational energy changes affect the skin?
Infrared radiation is sensed as heat- This radiation makes bonds in the chemicals in the skin vibrate more energetically. The molecules have more kinetic energy-so you feel warmer.
Vibrational Energy- explain how vibrational energy changes can affect small molecules such as HCl?
The HCl molecule can occupy only certain fixed levels of vibrational energy
When it receives more energy its vibrational energy increases and it moves up to a new fixed energy level.
Vibrational Energy- What does the specific/ quantised energy value absorbed depend on, explain using the carbon-halogen bonds?
The bond
E.g. the C-F bond is stronger than the C-Br bond (F more electronegative/ closer to positive nucleus) so C-F bonds must absorb infrared radiation of a higher energy (and so frequency) to make the C-F bond vibrate than to make the C-Br bond vibrate
Rotational Energy- When the rotational energy of a molecule changes what happens?
Rotation of the molecule as a whole
Rotational Energy- How many joules of energy are required, where in the electromagnetic spectrum is this found?
1x10-22 to 1x10-20J spacing between rotational energy levels/ needed found in the microwave part of the spectrum
Rotational Energy- Why do rotational energy changes require lower frequencies of electromagnetic radiation than vibrational energy changes?
Changes in rotational energy correspond to a lower energy than vibrational energy changes and so a lower frequency part of the EM spectrum in the microwave region
Translational Energy- What amount of energy is required for translational energy changes, where in the electromagnetic spectrum is this found?
Very low energy required – this happens continuously by molecules absorbing radio/ low end microwave parts of the electromagnetic spectrum
Translational Energy- how large is the spacing between translational energy levels?
The spacing between translational energy levels is even smaller than for rotational energy changes
When a small molecules e.g. chlorine absorbs electromagnetic radiation describe the three possible things which can happen?
Depending on the amount of energy/ frequency of radiation the chlorine molecules becomes excited then three things can happen:
1. Electrons are excited to a higher energy level – this is why chlorine is a green colour, Cl2 absorbs visible light of such a frequency that the remaining unabsorbed light looks green. The chlorine molecule then releases this energy and returns to its original state/ energy level.
2. Photodissociation- Higher energy radiation used, molecular absorbs so much energy that the bonding electrons can no longer bond the atoms together (bonds break) which forms radicals- called photodissociation
3. Ionisation- Very high energy photons- molecules acquire so much energy that an electron is able to leave- forming an ionised molecule, need very very high energy as this requires skipping many energy levels
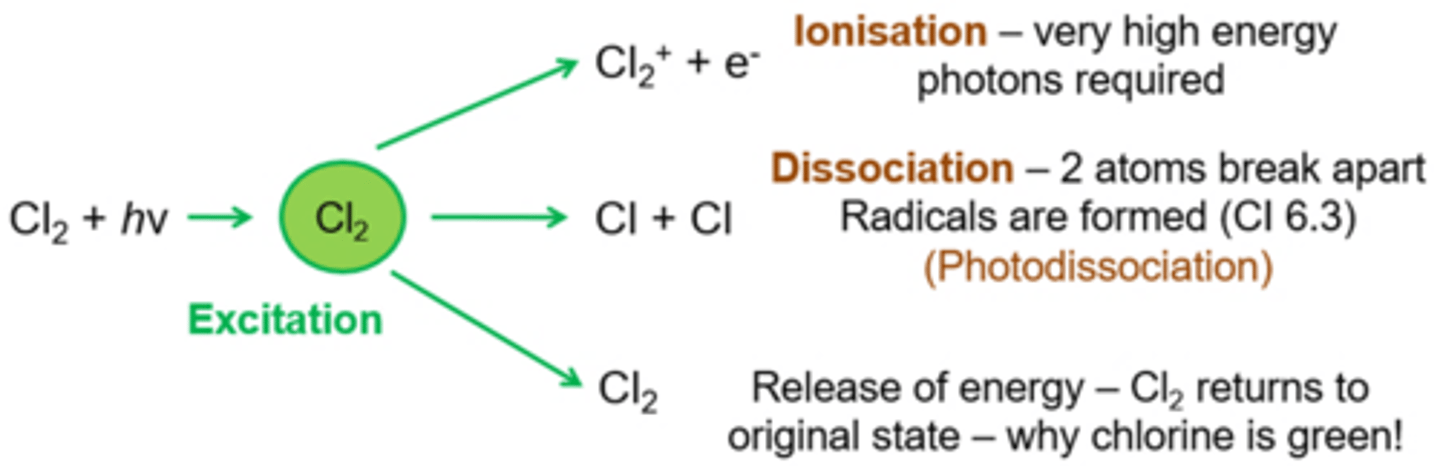
Define photodissociation
Breaking a bond in the presence of light
Define radical?
Molecules/ atoms with at least one unpaired electron, usually very reactive, formation may lead to further chemical reactions
How is energy transferred from one molecule to another?
By collision
EXAM TIP- Why should you use wavelength rather then frequency?
Wavelength has a linear scale but frequency does not