metallic bonding
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
what are some general properties of metals?
Delocalised electrons
Poor insulator
High Density
Malleable
Ductile
Left of PT
What are some general properties of non-metals?
Brittle in solid state
Poor conductors of heat and electricity
Low density
Not malleable
Not ductile.
What causes a substance to be more malleable, or soft?
Uniform layers within the structure of the atoms, allowing the atoms to slide over one another, causing the substance to be soft.
What type of layering within atoms causes the substance to be less malleable and more dense?
If the atoms sit in the ‘gaps’ of the previous row, instead of on top, meaning you get more atoms in the space available, and the layers don’t slide as easily
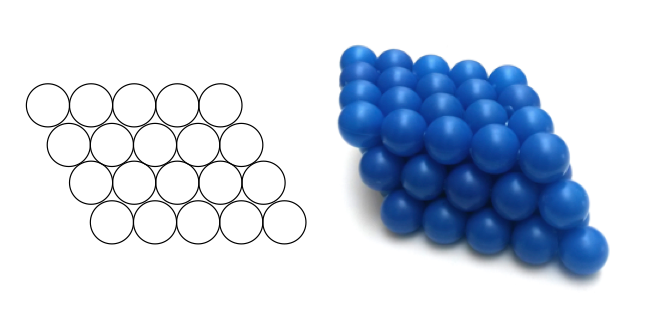
How can alloys make a metal more hard?
Alloys can make a metal harder by introducing different atoms that disrupt the arrangement, distorting the layers in the atoms, meaning these layers don’t slide as easily