12UBIO STUDY GUIDE UNIT 1 L8-L15
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
What are carbohydrates made up of? How are they formed?
Carbohydrates are macromolecules made up of simple sugar monomers, monosaccharides, and are made up of carbon, hydrogen and oxygen in a 1:2:1 ratio, and carbohydrates are formed from polymers, which are monomers linked together.
Classify the different types of carbohydrates.
There are simple carbohydrates which consist of monosaccharides and disaccharides, and complex carbohydrates which consist of oligosaccharides and polysaccharides.
What are Monosaccharides?
Monosaccharides: Single ringed molecules that have carbon, hydrogen and oxygen in a 1:2:1 ratio and at least two hydroxyl groups, and an aldehyde [H-C=O] or ketone [C=O]. group.
An example of monosaccharides are glucose, fructose, and galactose.
What are disaccharides?
Disaccharides: Two monosaccharide units linked together. They are soluble and able to be transported into blood easily, quickly raising blood sugar levels. however, there is no direct entry into the blood as the disaccharides must break down into enzymes.
An example of disaccharides are sucrose and lactose
What are olgiosaccharides?
Complex carbohydrate with 3-10 sugar units linked together. The sugars are linked together by covalent bonds, specifically glycosidic bond, which is a covalent bond that links sugar molecules to another group, forming the structural backbone of ogliosaccharides.
What are polysaccharides?
Polysaccharides are formed when many simple sugar units are linked together to form a polymer through polymerization. They are made with glycosidic bonds and take time to get into the bloodstream as they need to be broken down into enzymes. Because of this, instead of being used for immediate energy, polysaccharides are used to store energy (ATP).
Whats are examples of complex carbohydrates?
1. Starch - found in plants & made up of glucose
2. Glycogen - storage of glucose in animals
3. Cellulose - structural polysaccharide for plant wants
4. Fiber
What does our body use carbohydrates for?
Our body uses carbohydrates to provide energy for bodily functions since they act as an energy source for controlling blood-sugar levels, insulin, and metabolism. This carbohydrates are able to get into our bloodstream quickly after consumption due to their small size and polar function groups on it.
How is glucose an example of carbohydrates?
Glucose [C6H12H6]is an example of a monosaccharide and carbohydrate as it has carbon, hydrogen and oxygen in a 1:2:1 ratio, contains at least 2 hydroxyl bonds, and an aldehyde group. Glucose is hydrophillic, and because it is polar, it can dissolve into blood easily.
Glucose are starches such as potatoes, wheat and pasta.
others include:
vegetables
galactose - milk
sucrose - table sugar
What are examples of fructose? How is fructose an example of a carbohydrate?
fructose - honey, sugarcanes, -monosaccharides used to get into the bloodstream quickly to increase blood sugar levels, and it can be beneficial when ones blood sugar levels are low
Differentiate between condensation/dehydration and hydrolysis reactions.
Condensation/Dehydration reactions are reactions that build larger molecules from smaller ones while producing a water molecule. The reactions are anabolic since you're taking small molecules to form a larger one
Hydrolysis reactions are reactions where water is used as a reactant to split larger molecules into smaller ones, and because you're taking a large molecule and breaking it down, it is a catabolic reaction where enzymes are used to speed up the process
What is a good carb? What is a bad carb?
Good carbohydrates are those that are closest to its original form, such as brown rice & bananas. Bad carbohydrates are those that are processed and refined, such as cakes, white rice, cookies & flour.
What are lipids? What are they made up of? How are they formed?
Lipids are a class of greasy, oily, or waxy non-polar water-insoluble compounds. They're made up of carbon, hydrogen, and oxygen in a 1:1:1 ratio, formed with glycerol and 3 fatty acids.
What are fatty acids?
Fatty acids are made of a backbone of carbon atoms (up to 36) with a carboxyl [H-O] group at the end of the chain.
Describe saturated & unsaturated fats.
Saturated fats raise blood cholesterol levels, have a linear shape, are solid at room temp, and originate from animal sources.
Unsaturated fats lower blood cholesterol levels, have a bent shape, and are liquid at room temp.
classify types of lipids.
There are lipids with fatty acids, which are triglyceride, phospholipids and wax.
There are lipids without fatty acids, which are steroids & cholesterol
What is a triglyceride?
Triglyceride: glycerol joined to 3 fatty acid "tails". They are the largest class of non-polar lipids and contain twice the stored energy as carbohydrates of the same mass
What are phospholipids?
Phospholipids consist of a phosphate head, making it polar and hydrophobic. It is attached to glycerol and two fatty acid tails that wil avoid and not dissolve in water
What is wax?
Wax is a large lipid made from long fatty acid chains linked to alcohols or carbon rings. It is hydrophobic, and extremely non-polar. Wax has a firm, bendable consistency and has a function of water resistance and protection
What are steroids?
Small lipids without fatty acids that have four hydrocarbons rings fused
What is cholesterol?
A lipid without fatty acids that is an important steroid since it is a structural component of cell membranes and functional groups.
What does our body use lipids for? Provide specific examples.
Lipids are used in our body as they control what goes in and out of our cells. They help with transporting and storing energy, absorbing vitamins and making hormones.
Why are lipids used as lipoproteins?
Because fats and cholesterol cannot dissolve in water or blood, the body packages both fat and cholesterol into tiny protein covered particles, lipoproteins. Lipoproteins can transport excess lipids in blood and tissues to the liver for disposal, which is beneficial as it lowers blood cholesterol.
What are high density lipoproteins for? [HDL]
They transport excess lipids in blood and tissues to liver for disposal - "good" (lower blood cholesterol)
What are low density lipoproteins used for?
They transport lipids from liver through blood to cells - "bad" (raise blood cholesterol)
What types of fats are good for you? Bad for you? Why?
Good fats are unsaturated cis fats as they lower disease risk, help lower cholesterol levels, and build stronger cell membranes in the body. Foods high in good fats are vegetable oils, nuts, seeds, & fish.
Bad fats are saturated trans fats because they increase disease risk, even when eaten in small amounts. Foods containing trans fats are primarily in processed foods, and excessive intake raises bad cholesterol. An example of food high in bad fats is margarine
What are proteins made up of? How are they formed?
Proteins are polymers of amino acids which are composed of a central carbon atom linked to an amino group, a carboxyl group [COOH], hydrogen atom, and a side chain/R-group. Proteins are formed by the linking of amino acids based on the information encoded in the DNA.
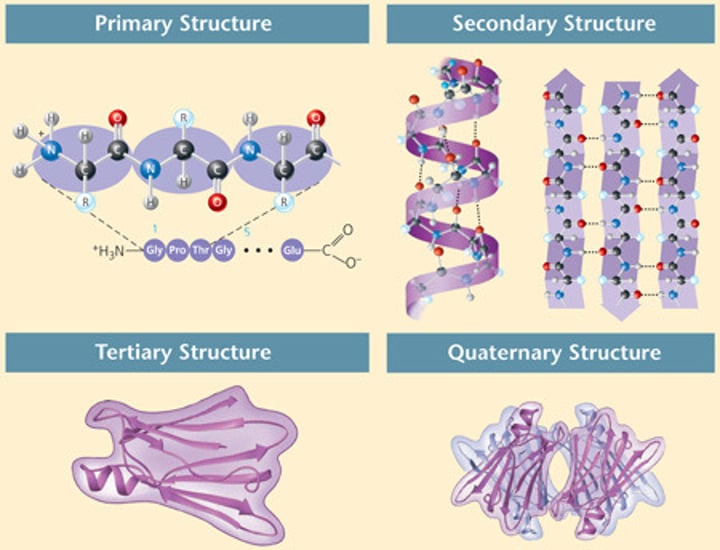
Discuss the levels of protein structure.
Primary Structure: linear sequence of amino acids in a polypeptide chain
Secondary Structure: • portions of repeated coils or folds caused by hydrogen bonding between adjacent amino acids.
• Polypeptide chain develops an Alpha helix (delicate coil) or a Beta pleated sheet (side by side alignment of amino acid chain - provides strength)
Tertiary Structure: 3D shape due to the interaction of R-groups. They interact to form ionic and H bonds, disulphide bridges, and hydrophobic interactions.
Variety of bonding can happen of all types which result in a 3D shape.
• Disulfide bridge Sulfur-Sulfur / S-S covalent bond stabilize shape of protein
Quaternary Structure:
• two or more polypeptides held together by the same forces above or containing an inorganic prosthetic group. Creates a very specific 3D shape (globular) with unique surfaces and pockets.
What does our body use proteins for? Provide specific examples.
Our body uses proteins for structure, as they provide support for our cells, repairing and making new cells. Our body uses proteins to build and repair muscles and bones, to make hormones and enzymes to catalyze reactions, and as an energy source.
What are nucleic acids made up of? How are they formed?
The building blocks of nucleic acids are nucleotides. Nucleotides consist of a 5-Carbon sugar, a phosphate group, and a nitrogenous base. They're formed by condensation reactions
Compare DNA and RNA
DNA and RNA have different sugars, base compositions, and number of strands
DNA: DNA is double stranded & has the pentose sugar of Deoxyribose. Base consists of Adenine, Guanine, Cytosine, Thymine.
RNA: RNA is single stranded & has the pentose sugar of Ribose. Base consists of Adenine, Guanine, Cytosine, and Uracil.
What does our body use nucleic acids for? Provide specific examples.
Nucleic acid is used in our body as replicators to copy genetic codes, and to store and create genetic information. For example, DNA encodes the information cells need to create proteins.
What are purine and pyrimidines
They are nitrogenous bases that make up the two different nucleotides in DNA and RNA. Purines (adenine and guanine) are two-carbon nitrogen ring bases while pyrimidines (cytosine and thymine) are one-carbon nitrogen ring bases
Identify the molecules of life based on their functional groups.
Carbohydrates - ketone [OH] ]or aldehyde [C-O=H]
Lipids - R-COOH (carboxyl group), long hydrocarbon chain, & ester linkages, (C double bonded to an O, single bonded to an O) [C=O-O] (if triglyceride)
Proteins - The R group makes one amino acid different from another. Amino acids differ only in their R-group. Carboxyl group [COOH], & hydrogen atom
Nucleic Acids - 5-Carbon sugar, a phosphate group, and a nitrogenous base.
→ Nitrogenous bases have no P group
Explain the difference between catabolic and anabolic reactions. Provide examples.
Catabolic Reactions - complex substance broken down into something less complex
Ex. Catabolic reactions are Hydrolysis [split larger molecules]
Anabolic Reactions - complex substance is built from something less complex
Simple + energy → complex
Condensation reactions would be anabolic [build larger molecules]
What is the first law of thermodynamics and an example of it in real life?
The total amount of energy in the universe is constant.
* energy cannot be created or destroyed, potential energy is just converted.
EX- Burning gasoline does not create new energy, potential energy is just turned into heat.
What is the second law of thermodynamics and an example of it in real life?
Energy in the universe is spontaneously flowing from areas of high energy content to low energy content
* Making less energy available for work to be done, as a result, the universe is becoming more disordered.
EX- You see a plate break into various pieces, but never see the plate putting itself back together on its own.
Define metabolism
Metabolism is the sum of all enzymes catalyzed catabolic and anabolic reactions in an organism. These reactions follow the laws of thermodynamics: (1. Total amount of energy in the universe is constant, 2. Energy in the universe is spontaneously flowing from higher to lower energy content)
Define Enthalpy (H)
Enthalpy (H): Total value of energy in a system
Define Entropy (S)
Entropy (S): measure of randomness of a system
Define Temperature (T)
Temperature (T): measure of molecular motion
Define Gibbs Free Energy. What is the equation for this?
Gibbs Free Energy (G): energy in system that can do useful work
Change in free energy = Change in enthalpy - Product of temperature • change in entropy
ΔG=ΔH-TΔS
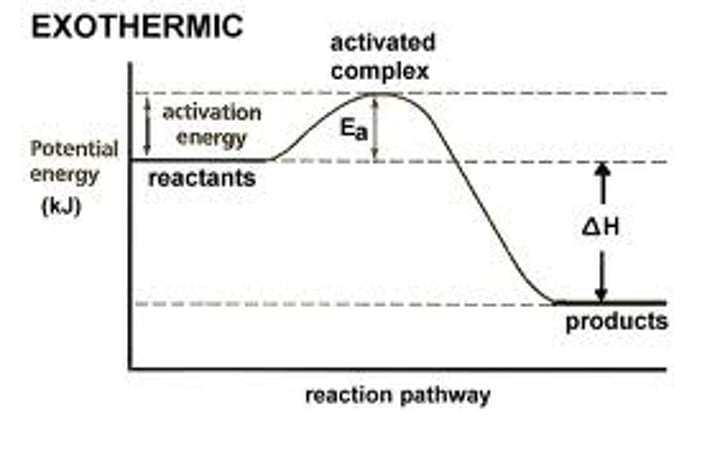
Define Exothermic reactions
Exothermic: Spontaneous, catabolic reactions that release energy. ΔH & ΔG is negative, T &, S are high, and the reaction is a catabolic
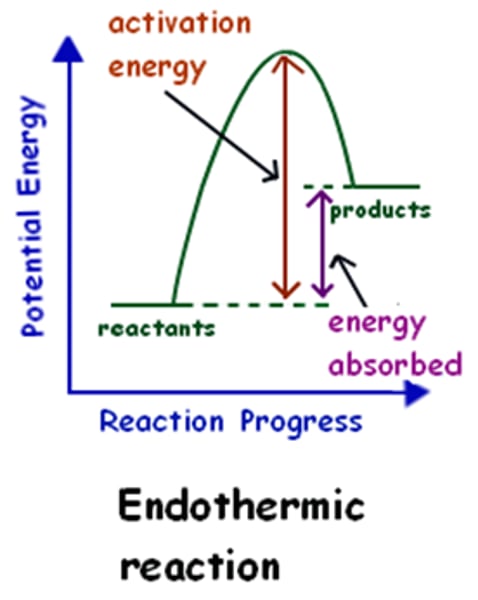
Describe endothermic reactions
Endothermic: Nonspontaneous, anabolic reactions where ΔG and ΔH is positive, while T & S go down.
What are spontaneous reactions?
Chemical reaction that will continue to completion without any further energy input once initiated (all chemical reactions need energy, once you put in the input of energy, it'll complete without any additional energy). An example is the oxidation of glucose.
What are non spontaneous reactions? Provide an example
Nonspontaneous reaction - reaction that can only continue as long as it receives a continual energy input. An example is the electrolysis of water
*Non Spontaneous reactions Require continual input of energy & use biochemical coupling
Explain the factors that determine if a reaction will be spontaneous or not.
SPONTANEOUS
EXOTHERMIC
- ∆G
- ∆H
HIGH T
HIGH ∆S
Catabolic
NON-SPONTANEOUS
ENDOTHERMIC
+ ∆G
+ ∆H
LOW T
LOW ∆S
Anabolic
Discuss the importance of biochemical coupling. Where does biochemical coupling occur?
Biochemical coupling is important as it allows controlled use of energy in nonspontaneous reactions. Nonspontaneous reactions require continual input of energy, which is metabolically expensive, As a result, nonspontaneous reactions are paired with spontaneous reactions to grant free energy, to drive the reactions. This occurs on the surface of enzymes.
Provide an example of biochemical coupling.
An example of biochemical coupling is synthesizing an ATP molecule since its s nonspontaneous reaction that needs to be coupled to a spontaneous reaction.
What is activation energy?
Activation energy is the minimum amount of energy required for a reaction to occur.
how do enzymes operate to decrease the activation energy of a reaction?
Enzymes work to decrease activation energy by reducing the amount of energy needed for reactants to come together and react.
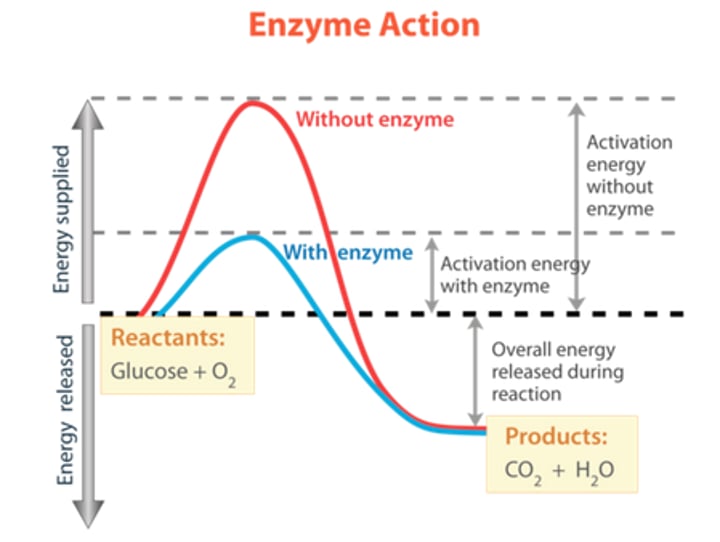
Draw and label a potential energy diagram for a reaction with the use of an enzyme and without, for exothermic & endothermic reactions
Enzymes are said to be specific in their function. What is it about enzymes, which leads to this specificity?
Enzymes are specific in their functions because of their shapes since they are globular protein catalysts made up of amino acids. Just like proteins, enzymes have different functions based on their 3-D shape, and have precisely shaped active sites specific to their function. As a result, enzymes can only catalyze single specific reactions.
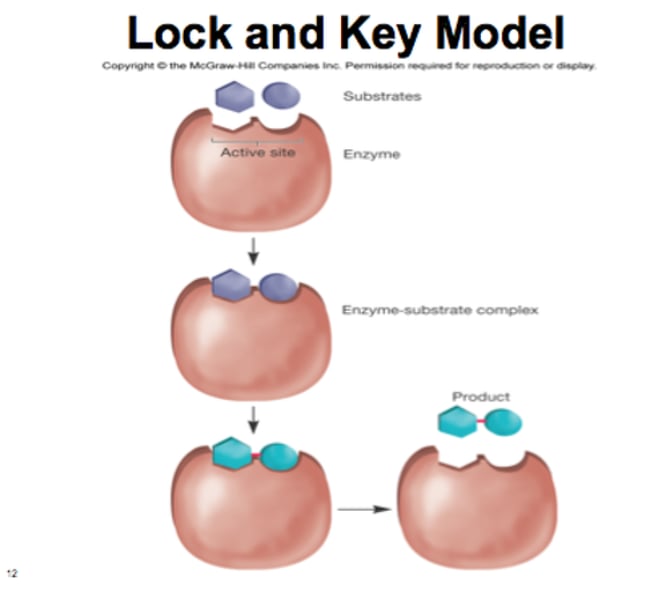
Describe the Lock and Key Model
The lock and key model was the early model of enzyme activity that proposes an exact fit between the enzyme and substrate. When the combined substrate was converted into product, the enzyme was left unchanged.
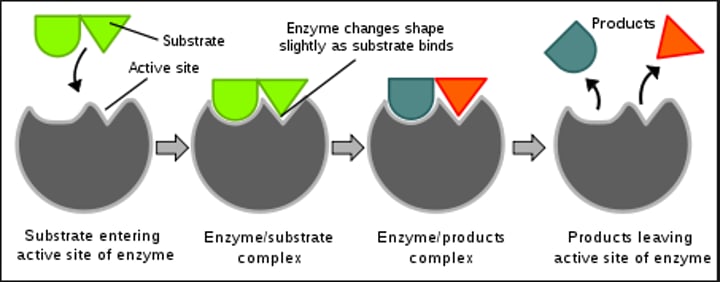
Describe the Induced Fit Model
The Induced Fit Model is the currently accepted model of enzyme activity. When a substrate molecule enters an active site & functional groups interact with the functional groups of the enzyme, this causes enzymes to change its shape to better accommodate the substrate. Afterwards, the enzyme then returns to its original shape.
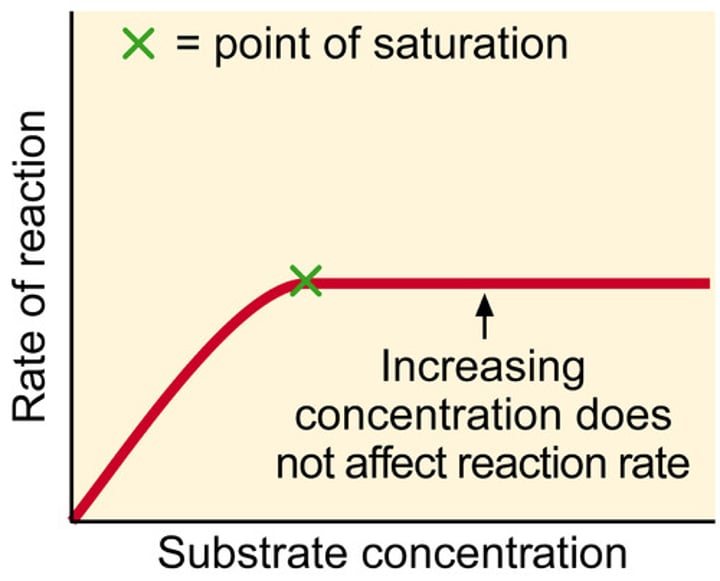
Describe the effect of ENZYME/SUBSTRATE CONCENTRATION on enzyme activity:
1. As number of substrate or enzyme molecules increases, so does chance of successful collision (increase)
2. Rate of activity ceases to rise as enzyme molecules are saturated with substrate (occupied) (constant)
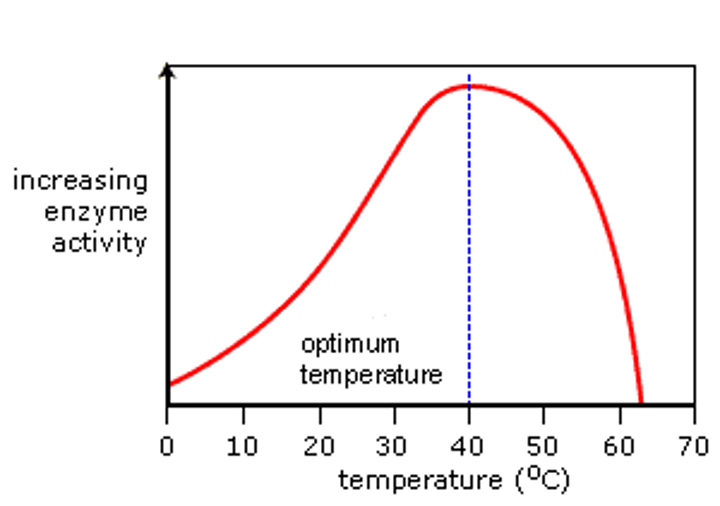
Describe the effect of TEMPERATURE on enzyme activity:
1. As temperature increases so does molecular motion of enzyme and substrate. Increases probability of successful collision (go up)
2. Low temps don't provide enough thermal energy to activate enzymes. High temp (40+) denatures enzymes - breaks H bonds(go down)
****Denaturation - Disrupt chemical bonds - change active site shape.
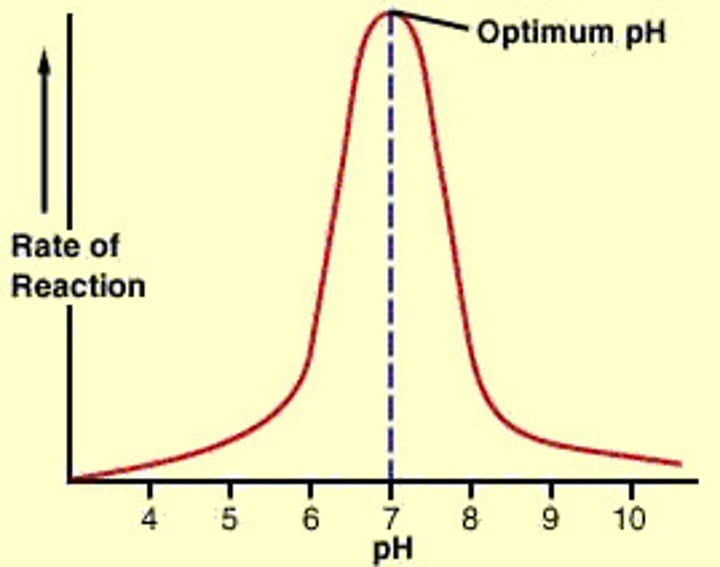
Describe the effect of pH on enzyme activity:
• Enzyme reactions typically occur in aqueous solutions
• Change of pH alters charge of enzyme - alters shape of active site and solubility, which lowers enzyme function
• Enzymes have an optimal pH range