Lesson I
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
Pharmaceutical care
Evidence based pharmacy
Meeting patient’s needs
Chronic patient care
Self medication
QA In pharmacy services
Clinical pharmacy
Pharmacovigilance
What are the new dimensions of Pharmacy Practice
Caregiver
Decision maker
Communicator
Manager
Life long learner
Teacher
Leader
Seven star pharmacist
RA 6675 Generic Dispensing Act
Rules and Regulations to Implement Prescribing Requirements under the Generics Act of 1988
LIST A (ANNEX 1)
Controlled Substances Act Classification
List of Pharmaceutical Products classified as Prohibited Drugs or Regulated Drugs by the Dangerous Drug Board
LIST B (ANNEX 2)
Controlled Substances Act Classification
List of Products Requiring Strict precaution in Prescribing, Dispensing, and Use
Use generic terminology
Adequate supply and lowest possible cost of generics
Encourage the use of generics
Emphasize the scientific basis of generics
Promote drugs safety
What are the main policies of Generics Act of 1988

A.O. No. 62 s. 1989
Rules and Regulations to Implement Prescribing Requirements under Generics Act of 1988 (RA 6675)
Where does this fall under
Generic prescribing
Is prescribing of drugs using their generic name(s) or generic terminology
Prescribing the patient’s /buyer’s choice from among generic equivalent
Generic name or terminology
is the identification of drugs and medicines by their scientifically and internationally recognized active ingredient(s), same dosage form and same strength as the prescribed drug.
Prescription
Is the written order and instruction of a valid registered physician, dentist or veterinarian to use a specific drug product for a specific patient.
Dispensing
Is the act by a validly-registered pharmacist of filling a prescription or doctor’s order on the patient’s chart.
Drug product
is the finished product form that contains the active ingredients, generally but not necessarily in association with inactive ingredients
Dangerous drugs
refer to either prohibited drugs or regulated drugs which require a special prescription form, the use which is monitored by the Dangerous Drugs Board
Prohibited drug
which includes opium and its active components and derivatives, such as heroin and morphine; coca leaf and its derivatives, principally cocaine; alpha and beta eucaine; hallucinogenic drugs, such as mescaline, lysergic acid diethylamide (LSD) and other substances producing similar effects; Indian hemp and its derivates; all preparations made from any of the foregoing; and other drugs, whether natural or synthetic, with the physiological effects of a narcotic drug
Regulated drug
which includes self-inducing sedatives, such as secobarbital, phenobarbital, pentobarbital, barbital, amobarbital and any other drug which contains a salt or a derivative of a salt of barbituric acid; any salt, isomer or salt of an isomer, of amphetamine, such as benzedrine or dexedrine, or any drug which produces a physiological action similar to amphetamine; and hypnotic drugs, such as methaqualone or any other compound producing similar physiological effects
Loose drugs
In dispensing to the buyer, drug products in unit doses or products which are not in their original containers but transferred to small bottles, tin cans, plastic and/or paper envelopes and Name of the patient: the like.
What is this
Px name
Generic name
Brand name
Manufacturer
Dosage strength
Expiry date
Instructions
Pharmacist name
For loose drugs, The pharmacist shall place legibly on the required drug outlets label the following information:
Partial filling
The following should be filled in what fillingg
True
True or false
The drugstore which completes the filling of the prescription shall keep the prescription in files.
False
True or false
Partial filling of prescription for drugs belonging to List A is allowed.
Simple prescription
with only one ingredient, those written for a single component or prefabricated product and not requiring compounding or admixture by the pharmacist.
Compound prescription
with more than one ingredient, those written for more than a single component and requiring compounding.
Dangerous drug prescription
Prescription for controlled substances contains a narcotic substance or other habit-forming drugs.
E-prescriptions
these are prescriptions transmitted to a pharmacy by computer.
Polypharmacy
with ten or more than two ingredients of the same therapeutic uses. Also called the shotgun preparation
Magistral prescription
is a prescription which is prescribed very often by the same doctor, of the same ingredients and compounded by the same pharmacist
Coded prescription
also called the blind prescription and consists of words, symbols, to represent the names of the drugs. This is an unethical practice.
OTC/Non-prescription drugs
Prescription/Ethical/Legend drugs
2 Broad Legal Classifications of Medications
Dangerous drug board
Provides special prescription form for practitioners authorized to prescribe dangerous drugs (which include both prohibited and regulated drugs) printed in security paper and subsequently numbered.
Blue - retained by physician
Yellow - retained by pharmacist
White - retained by patient
DDB-FORM 1-72 may be issued in three copies:

DDB Form 1-72
Only physicians whi S2 license can issue this
Recite
Answer recite
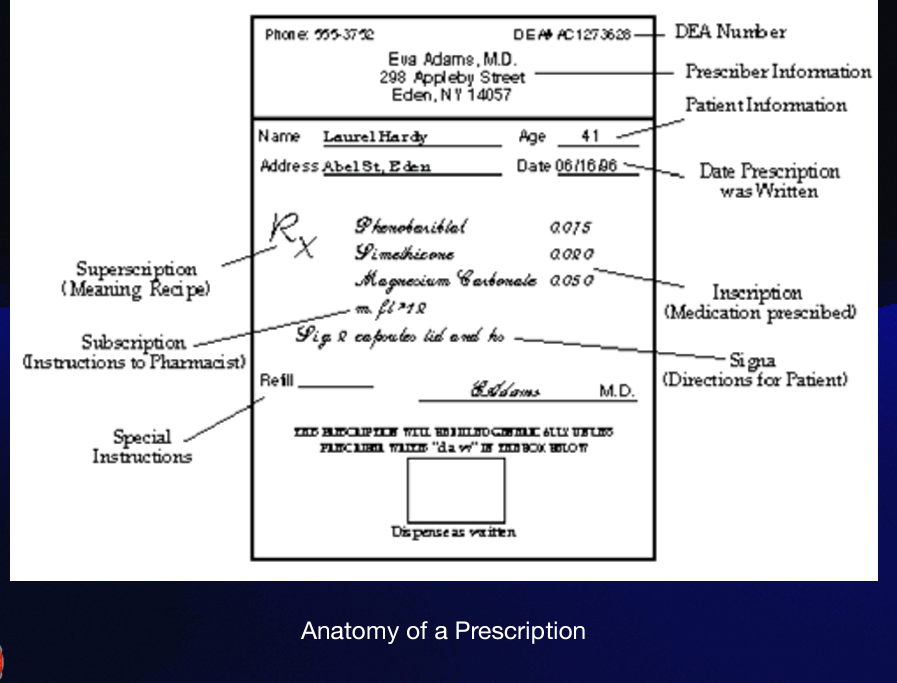
Praescriptus
The word "prescription" stems from the Latin term

Superscription
which consists of the heading where the symbol Rx (an abbreviation for recipe, the Latin for take thou ) is found. The RX symbol comes before the inscription.
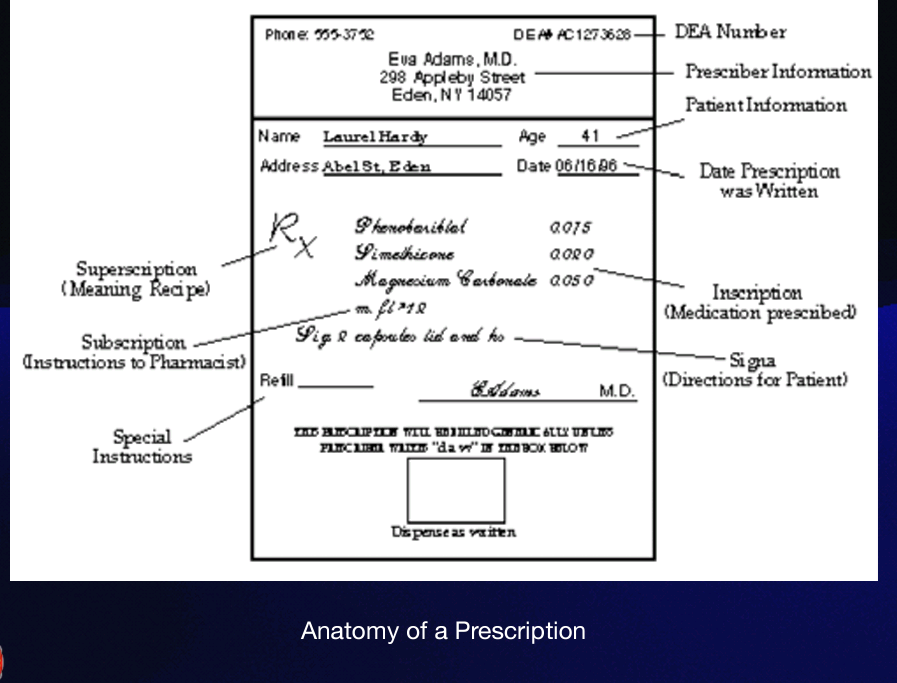
Inscription
is also called the body of the prescription, and provides the names and quantities of the chief ingredients of the prescription. Also in the inscription you find the dose and dosage form, such as tablet, suspension, capsule, syrup.
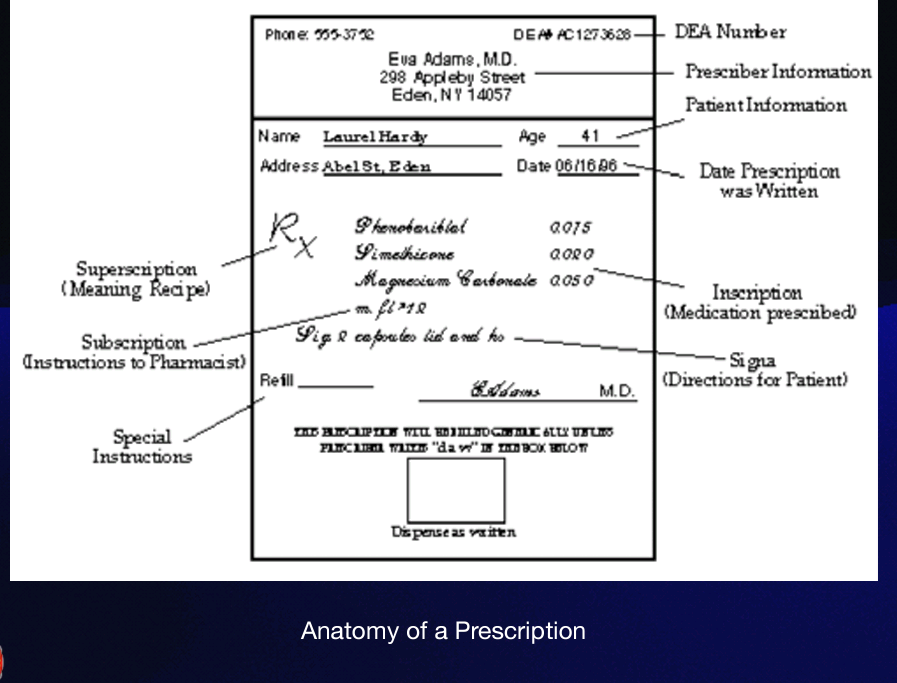
Subscription
which gives specific directions for the pharmacist on how to compound the medication. These directions to the pharmacist are usually expressed in contracted Latin or may consist of a short sentence such as: "make a solution," "mix and place into 10 capsules," or "dispense 10 tablets." However, that was in the old days. Today... doctors just name the pill!
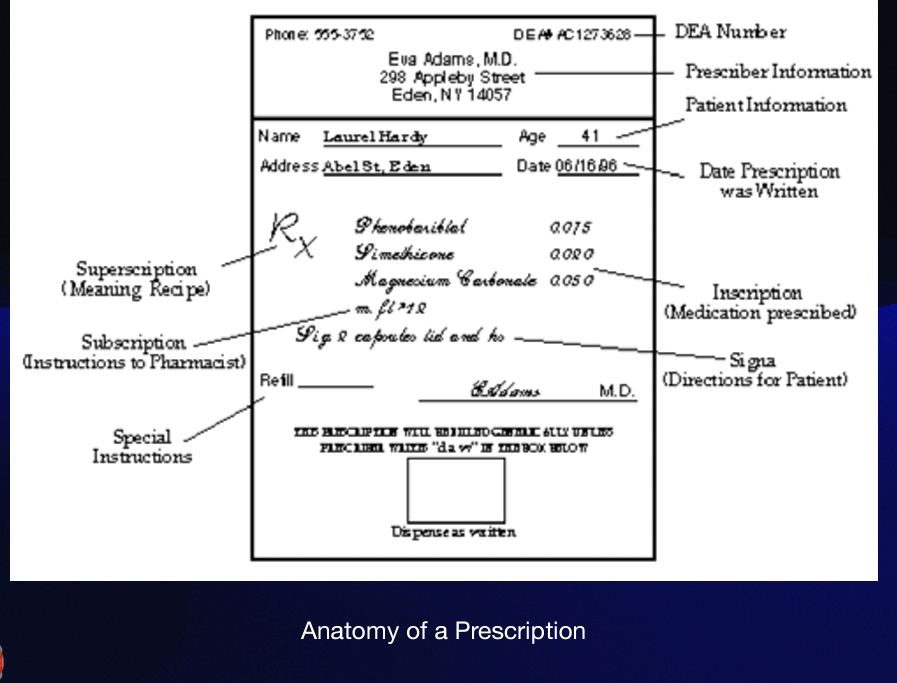
Signatura
(also called sig, or transcription), gives instructions to the patient on how, how much, when, and how long the drug is to be taken. These instructions are preceded by the symbol “S” or “Sig.” from the Latin, meaning "mark."
None or no refills
Instead of writing 0 in refills, use —
DEA (Drug enforcement agency) Registration number
— was implemented as a way to successfully track controlled substances from the time they are manufactured until the time they are dispensed to the patient.
Processing the prescription order
Receiving the prescription
Reading and checking the Rx
Labeling
Packaging
Recording
Filling
What are the prescription procedures
True
True or false
Out of the three prescription errors, only erroneous can be filed
True
True or false
Failure to report to the nearest DOH office cases of 3 incorrect prescriptions within 3 months after receipt of such prescriptions.
Generically equivalanet drugs
Are finished pharmaceutical products having the same active ingredients, same strength, and are of same dosage form.
Round vials
used primarily for solid dosage forms as capsules and tablets
Prescription bottles
used for dispensing liquids of low viscosity
Wide mouthed bottles
used for bulk powders, large quantities of tablets or capsules, and viscous liquids that cannot be poured readily from the narrow-necked standard Rx bottles
Dropper bottles
used for dispensing ophthalmic, nasal, otic, or oral liquids to be administered by drops
Applicator bottles
used for applying liquid mediation to a wound or skin surface
Oint jars and collapsible tubes
used to dispense semisolid dosage forms, such as ointments and creams
Sifter rop containers
used for powders to applied by sprinkling
Hinged lids or slide boxes
used for dispensing suppositories and powders prepared in packets
Aerosol containers
used for pharmaceutical aerosol products. They are pressurized systems dispensed by the pharmacist in the original container.