12-Aldehydes and Ketones
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
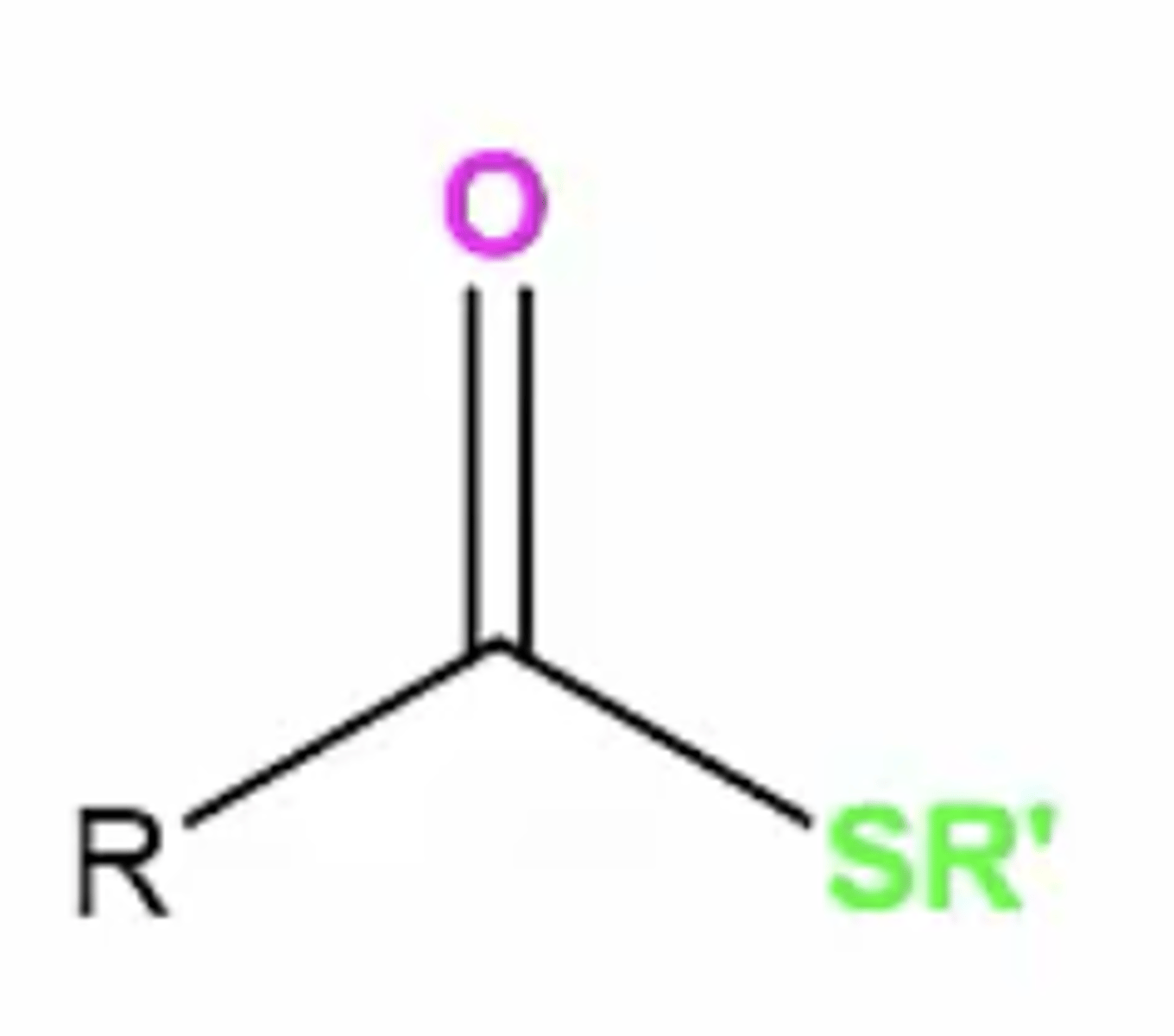
thioester
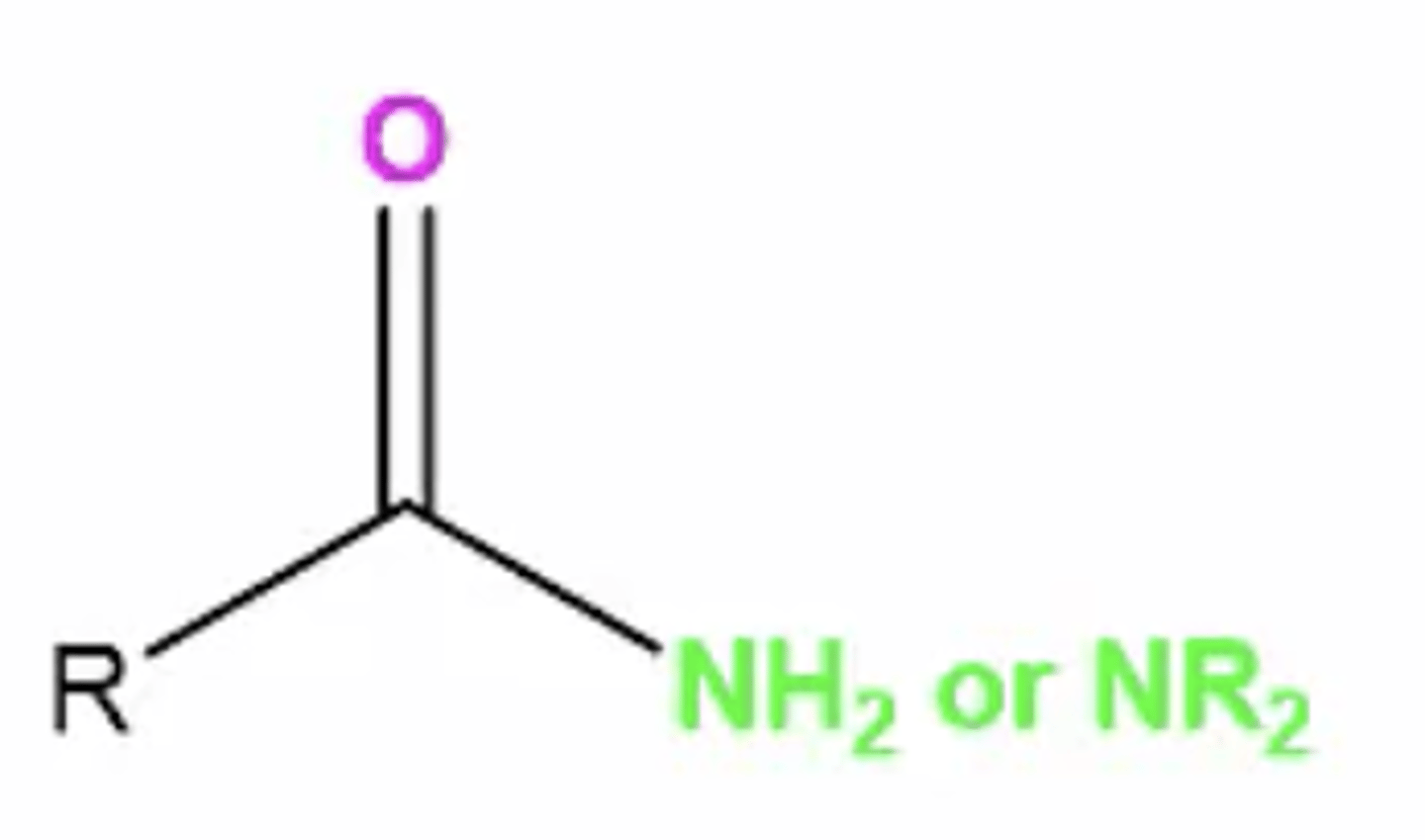
amide
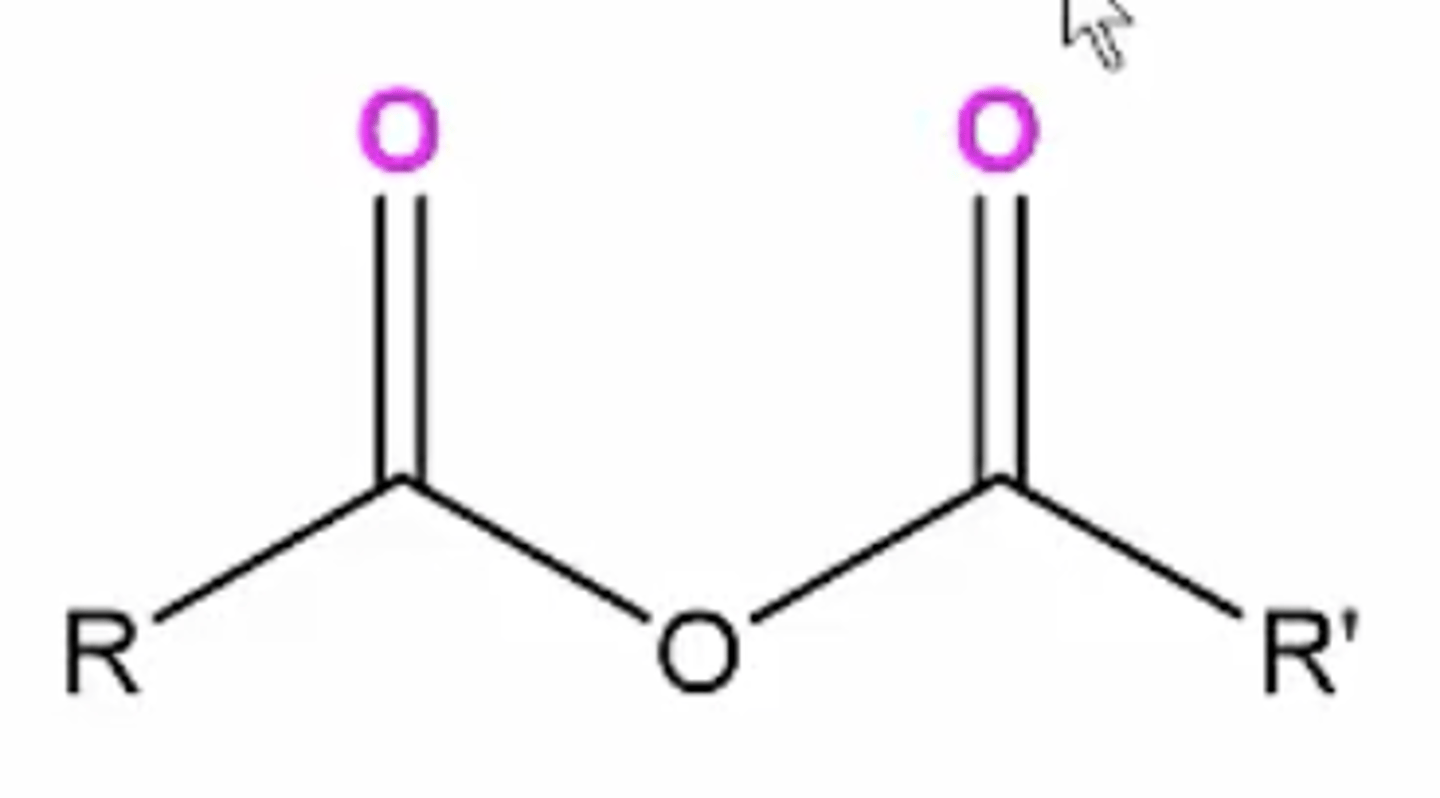
acid anhydride
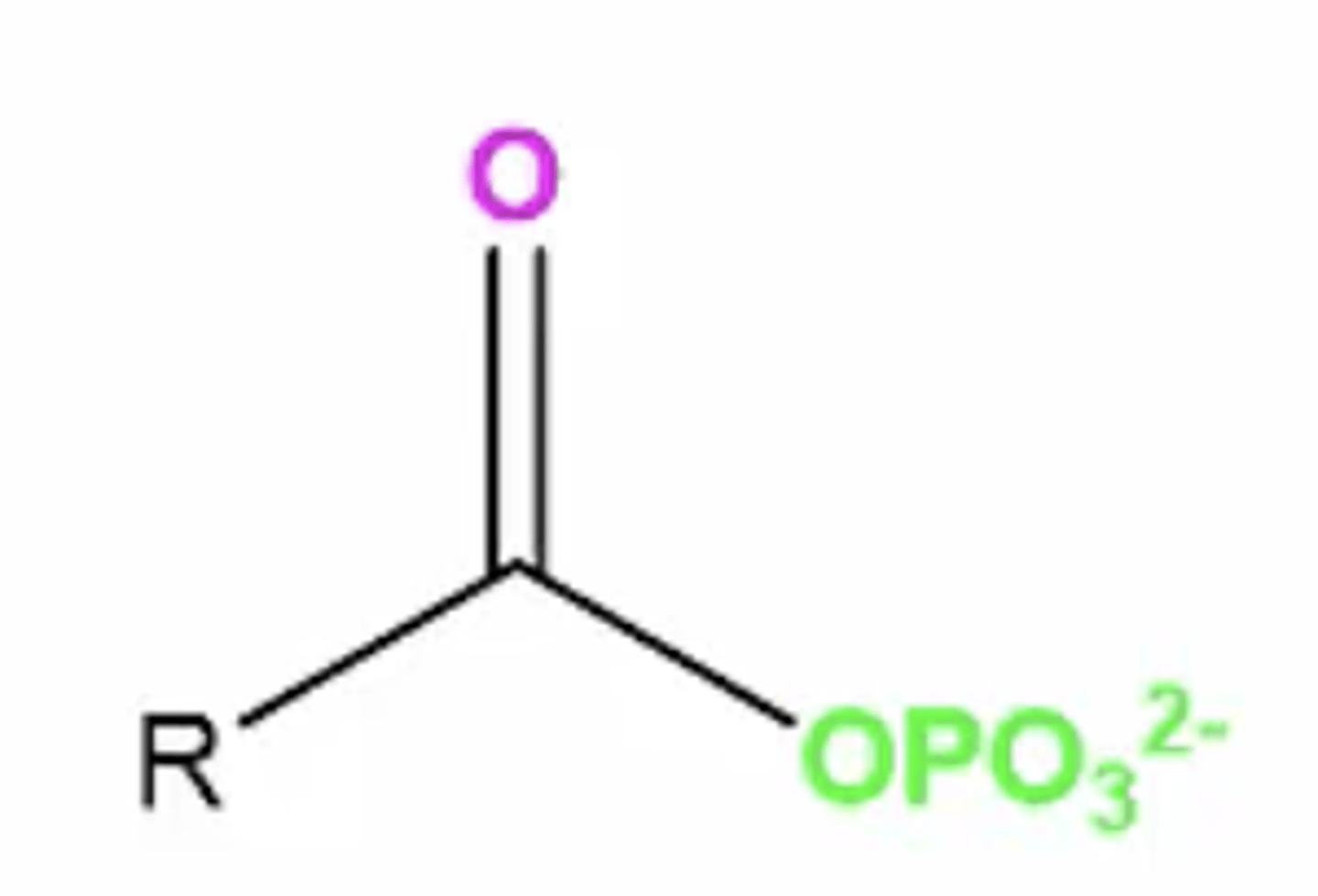
acyl phosphate
in general, how do carbonyl compounds react?
a nucleophile will kick the carbonyl compound in the crotch (attacking the carbonyl carbon)
-this produces a tetrahedral intermediate
-if the carbonyl compound is not an aldehyde or ketone, the negative charge on O will kick Y off as a leaving group
-if the carbonyl is an aldehyde or ketone, you must quench the rxn to protonate the O-
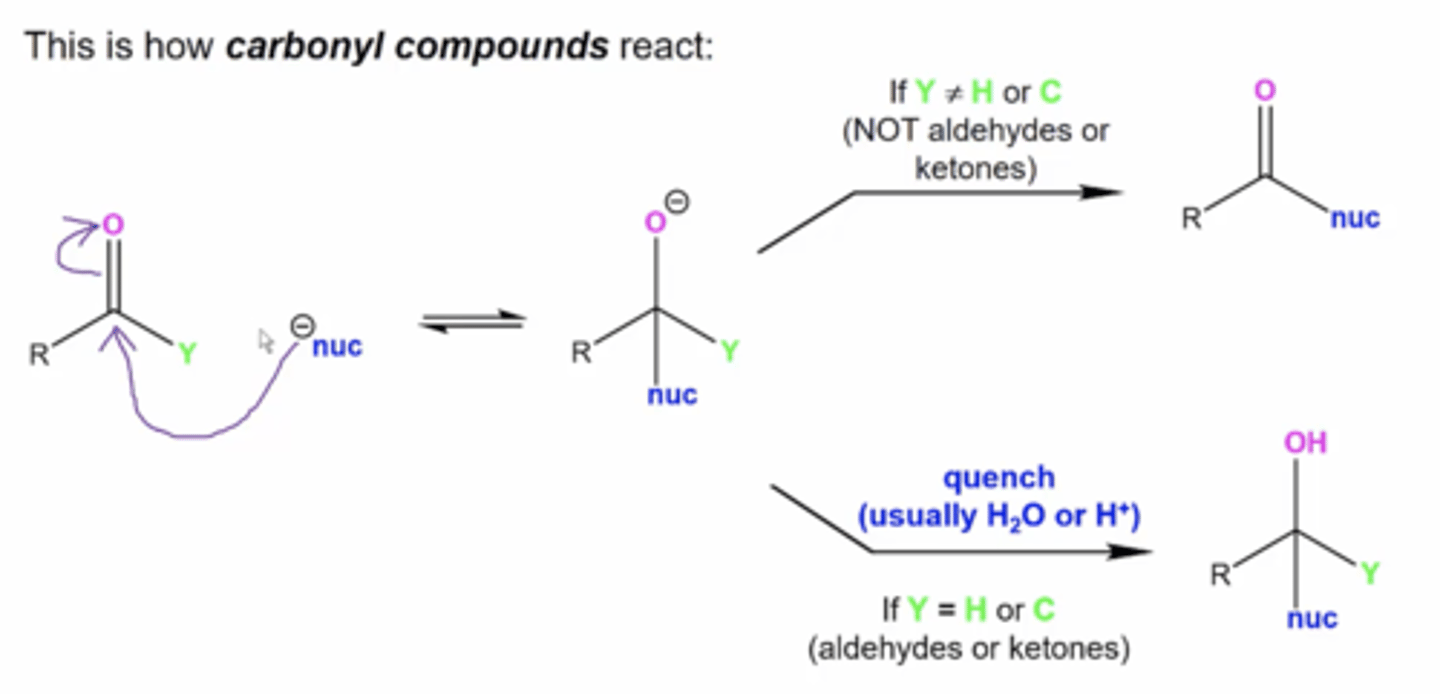
are aldehydes or ketones more reactive?
aldehydes because the hydrogen in an aldehyde is much smaller than a carbon in a ketone, so it's easier for the nucleophile to attack because there's less steric hindrance
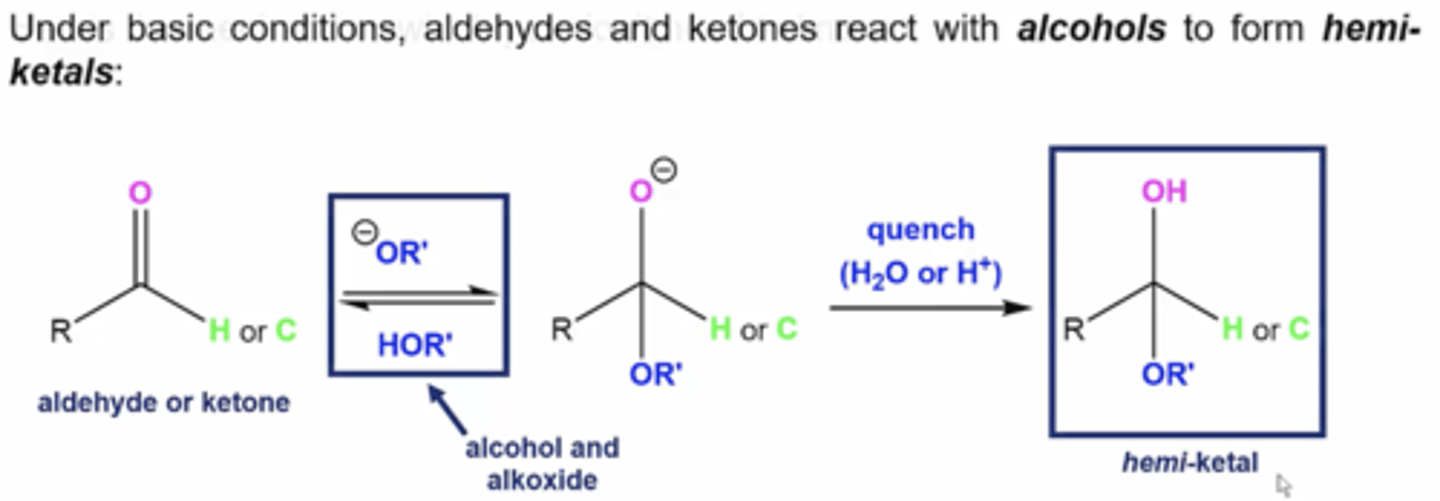
what happens if you react aldehydes and ketones with alcohols under basic conditions (use alkoxide base (OR-) and complementary alcohol solvent (HOR))?
form a hemiketal:
1. alkoxide base attacks carbonyl carbon to form a tetrahedral intermediate
2. quench reaction with H2O or H+ to protonate the O- from the intermediate to form a hemiketal
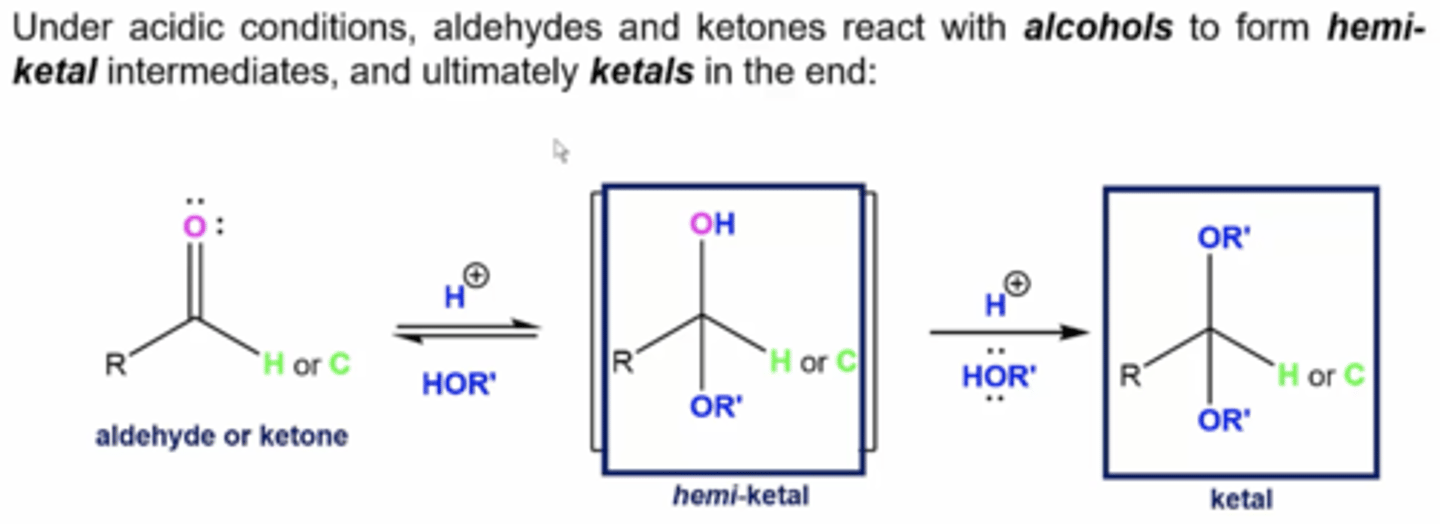
what happens if you react aldehydes and ketones with alcohols under acidic conditions (H+ with alcohol solvent (HOR))
form hemiketal intermediates, and ketals as final product:
1. H+ in alcohol protonates the carbonyl oxygen, breaking its double bond and forming a tetrahedral hemiketal intermediate
2. additional H+ ions will deprotonate the OH, turning it into an OR
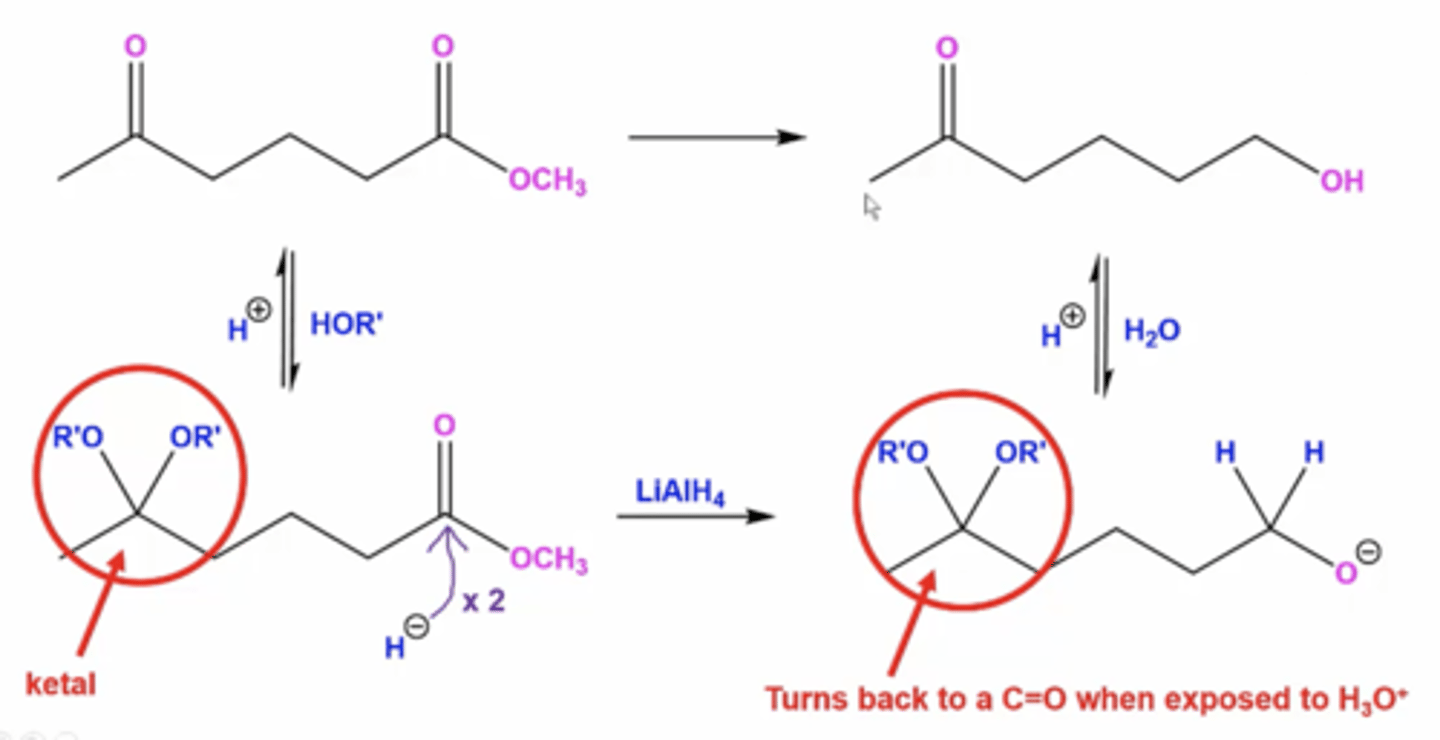
why are ketals used in synthesis reactions?
they can temporarily convert aldehyde/ketone C=O groups when you want to reduce some functional groups but not other aldehyde/ketones
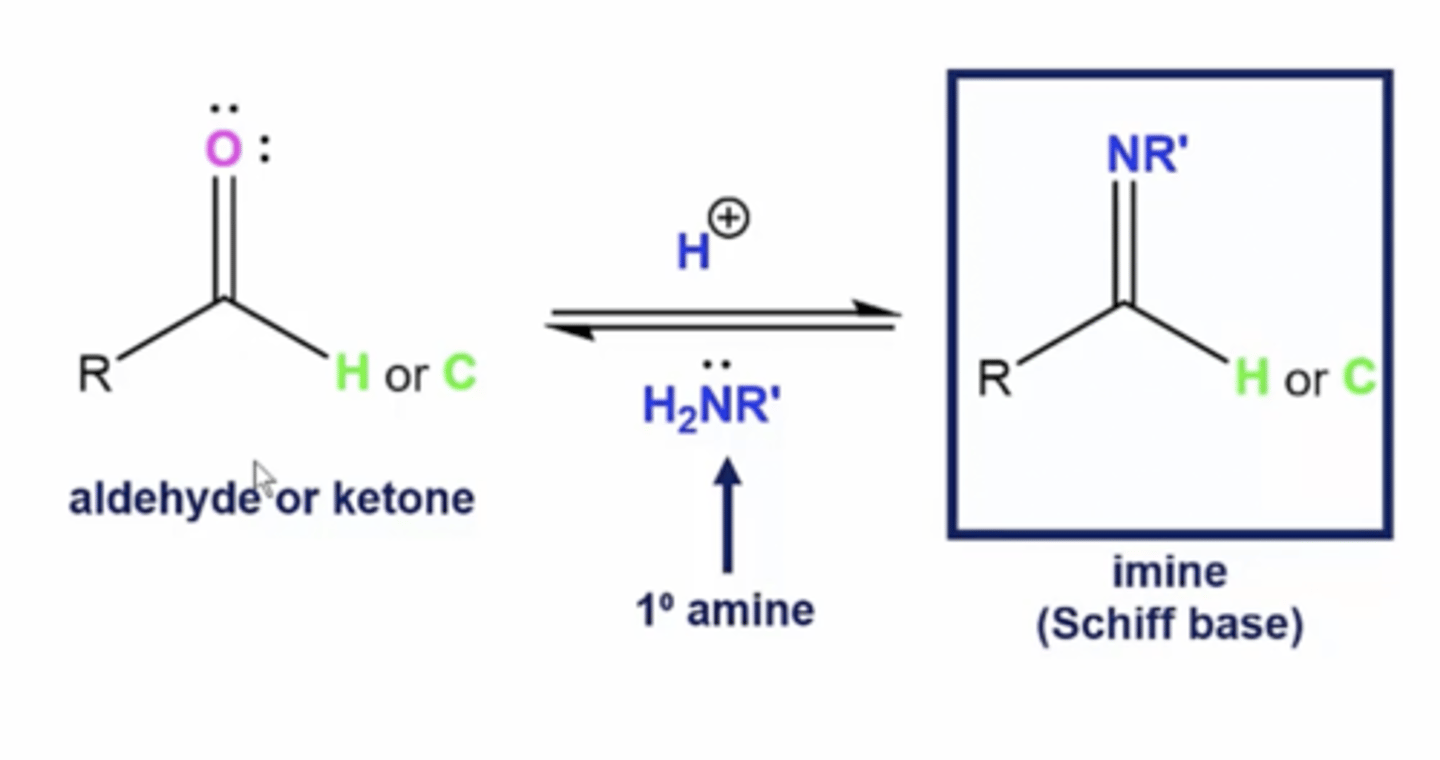
what happens when you react an aldehyde/ketone with a primary amine?
form an imine (schiff base)
1. the nitrogen of the amine will take the place of the carbonyl oxygen
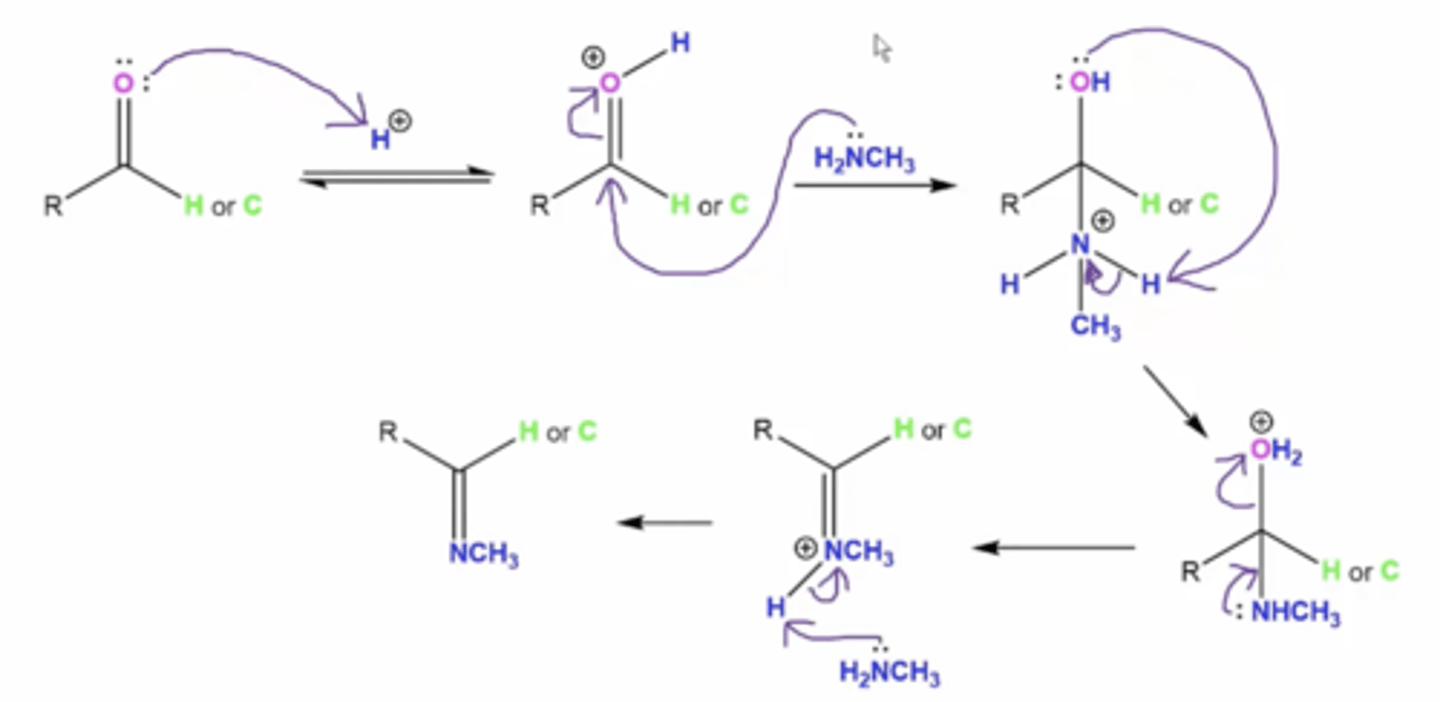
in general, what is the mechanism for reacting aldehydes/ketones with primary amines to form Schiff bases/imines
1. the carbonyl oxygen attacks an acidic H+ ion
2. the lone pairs from the amine's nitrogen attack the carbonyl carbon forming a very unstable tetrahedral intermediate with a positively charged N
3. the lone pairs on the carbonyl oxygen attack an H from the amine to neutralize the nitrogen's positive charge
3. lone pairs on nitrogen will drop down to form a double bond
4. lone pairs from excess amine will deprotonate one of the amine hydrogens to form final product
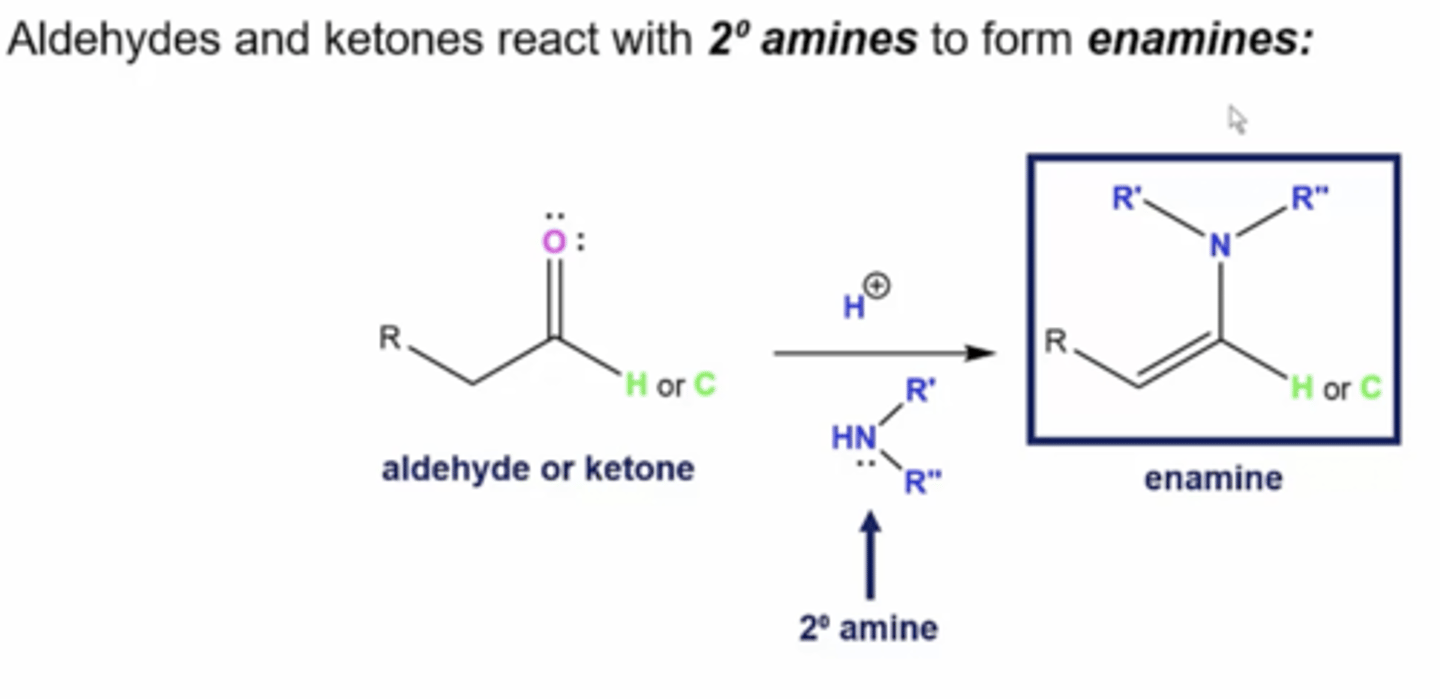
what happens when you react an aldehyde/ketone with a secondary amine?
you form an enamine (alkene+amine):
1. the nitrogen in the amine will take the place of the carbonyl oxygen, with a double bond on the most substituted carbon
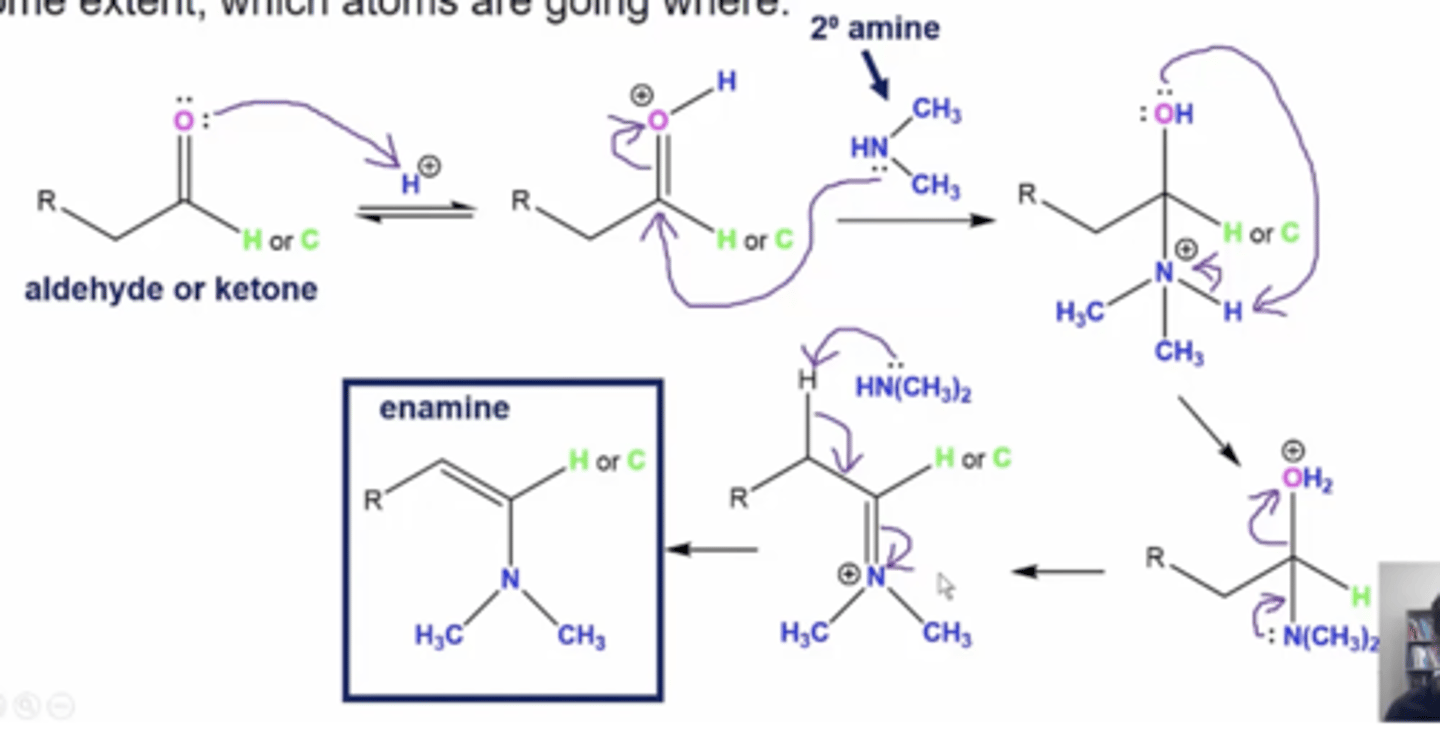
in general, what is the mechanism for reacting aldehydes/ketones with secondary amines to form enamines
1. the carbonyl oxygen attacks an acidic H+ ion
2. the lone pairs from the amine's nitrogen attack the carbonyl carbon forming a very unstable tetrahedral intermediate with a positively charged N
3. the lone pairs on the carbonyl oxygen attack an H from the amine to neutralize the nitrogen's positive charge
4. lone pairs on nitrogen will drop down to form a double bond and a positive charge on the nitrogen
5. a basic amine will deprotonate an alpha hydrogen, forming an alkene
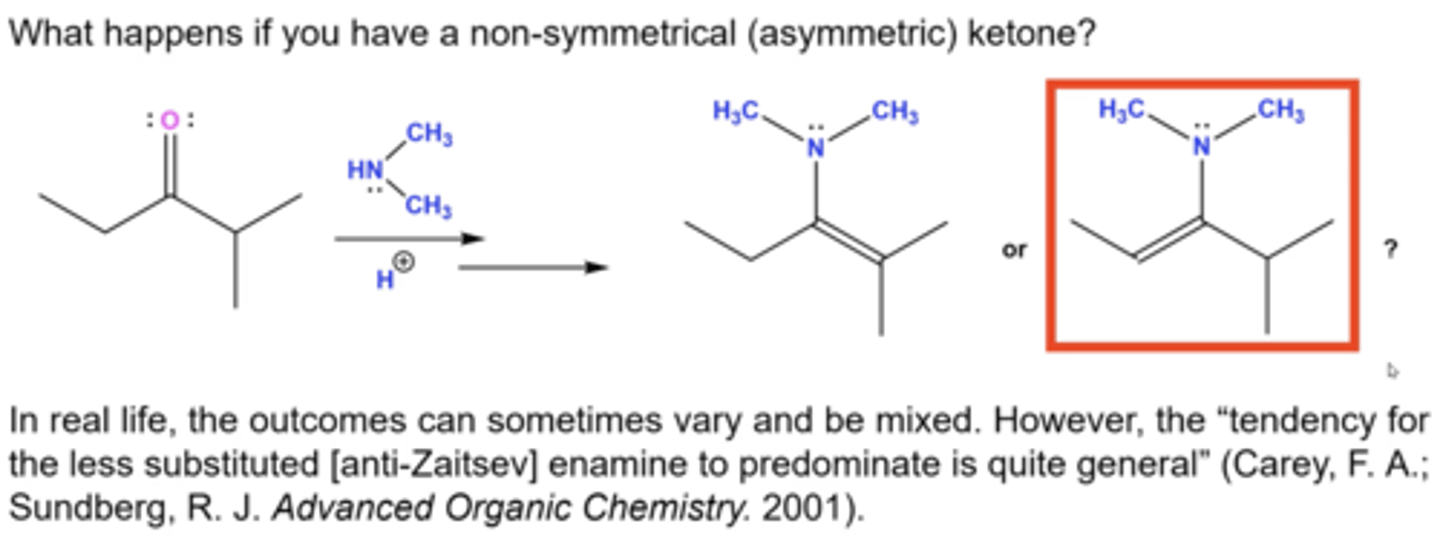
which product will be the major product when reacting an asymmetrical ketone with a secondary amine?
you'll form the anti-zaitsev (least substituted) product the most
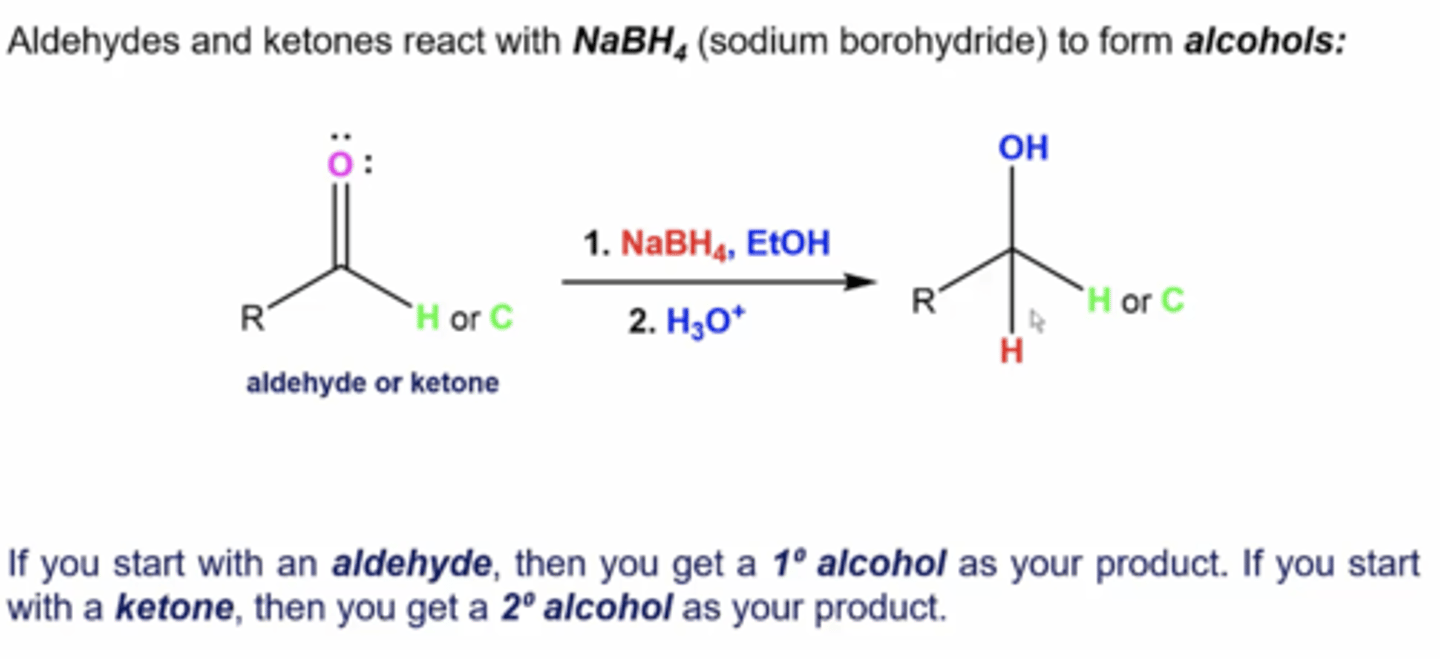
what happens when you react an aldehyde/ketone with this reactant:
1. NaBH4, EtOH
2. H3O+
you reduce the aldehyde/ketone into an alcohol (primary alcohols for aldehydes and secondary alcohols for ketones)
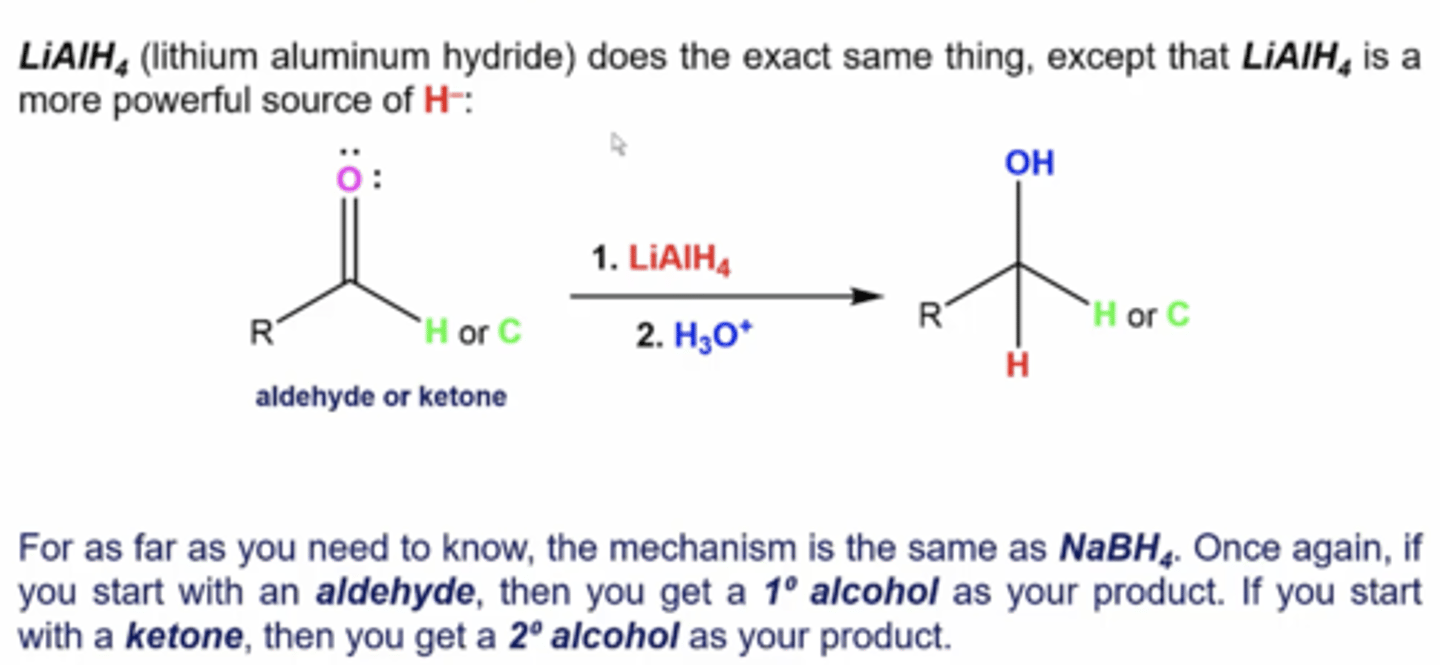
what happens when you react an aldehyde/ketone with this reactant:
1. LiAlH4
2. H3O+
you reduce the aldehyde/ketone into an alcohol (primary alcohols for aldehydes and secondary alcohols for ketones)
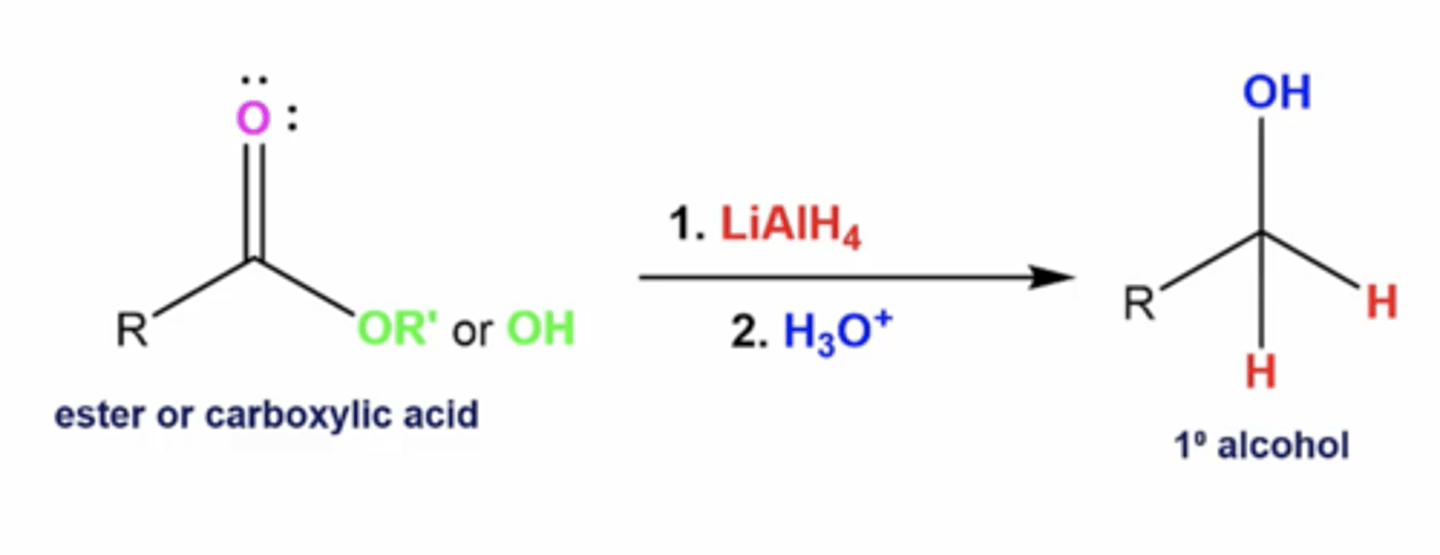
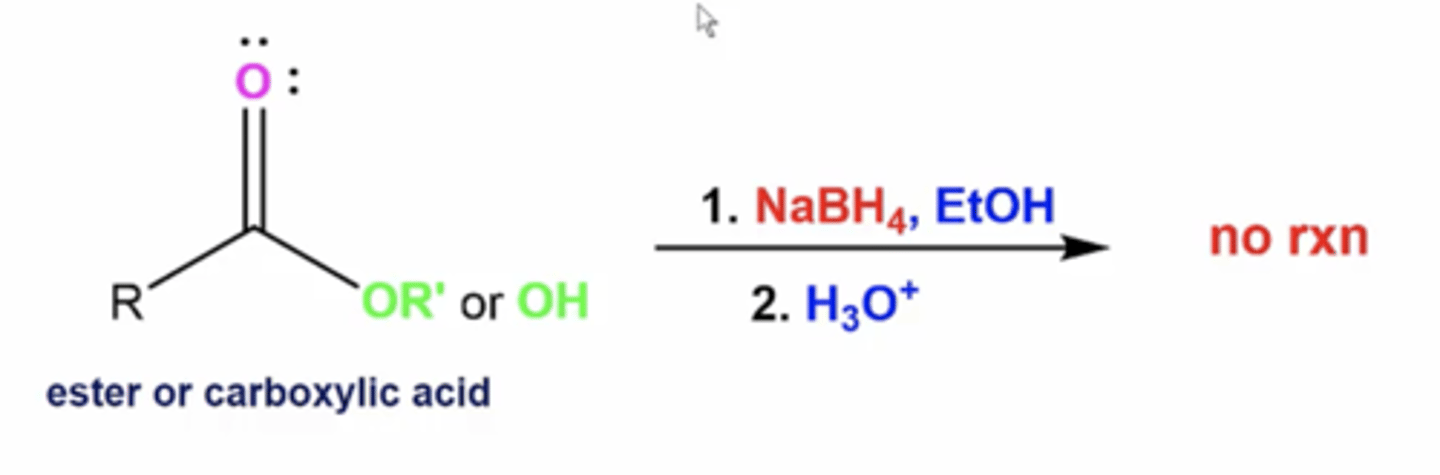
what happens when you react an ester/carboxylic acid with this reactant:
1. LiAlH4
2. H3O+
you reduce esters and carboxylic acids to primary alcohols
what happens when you react an ester/carboxylic acid with this reactant:
1. NaBH4, EtOH
2. H3O+
no rxn because NaBH4 isn't a strong enough protonator
what is a Grignard reagent?
R-MgX (hydrocarbon attached to metal and halide)
X=Cl, Br, or I
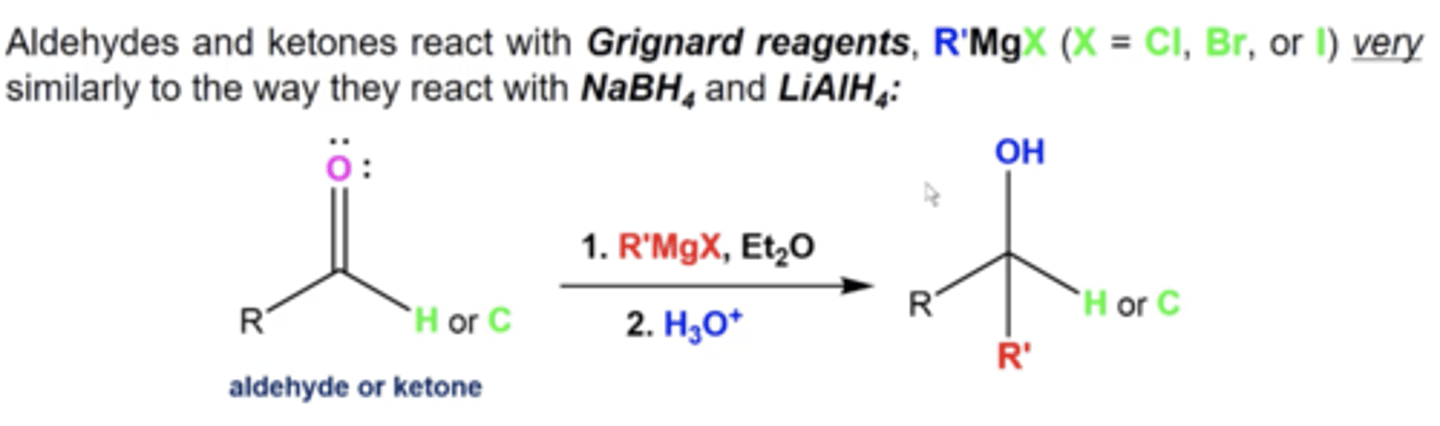
what happens when you react aldehydes/ketones to this reagent:
1. R'MgX, Et2O
2. H3O+
you add the R' from the Grignard and turn the carbonyl oxygen into an OH
-aldehyde = secondary alcohol
-ketone = tertiary alcohol
what is the mechanism to react aldehydes/ketones with grignards?
1. R- from Grignard attacks carbonyl carbon, which pushes up the double bond protons onto the carbonyl oxygen
-this forms a tertiary intermediate
2. the O- from the carbonyl is protonated by H3O to produce an OH
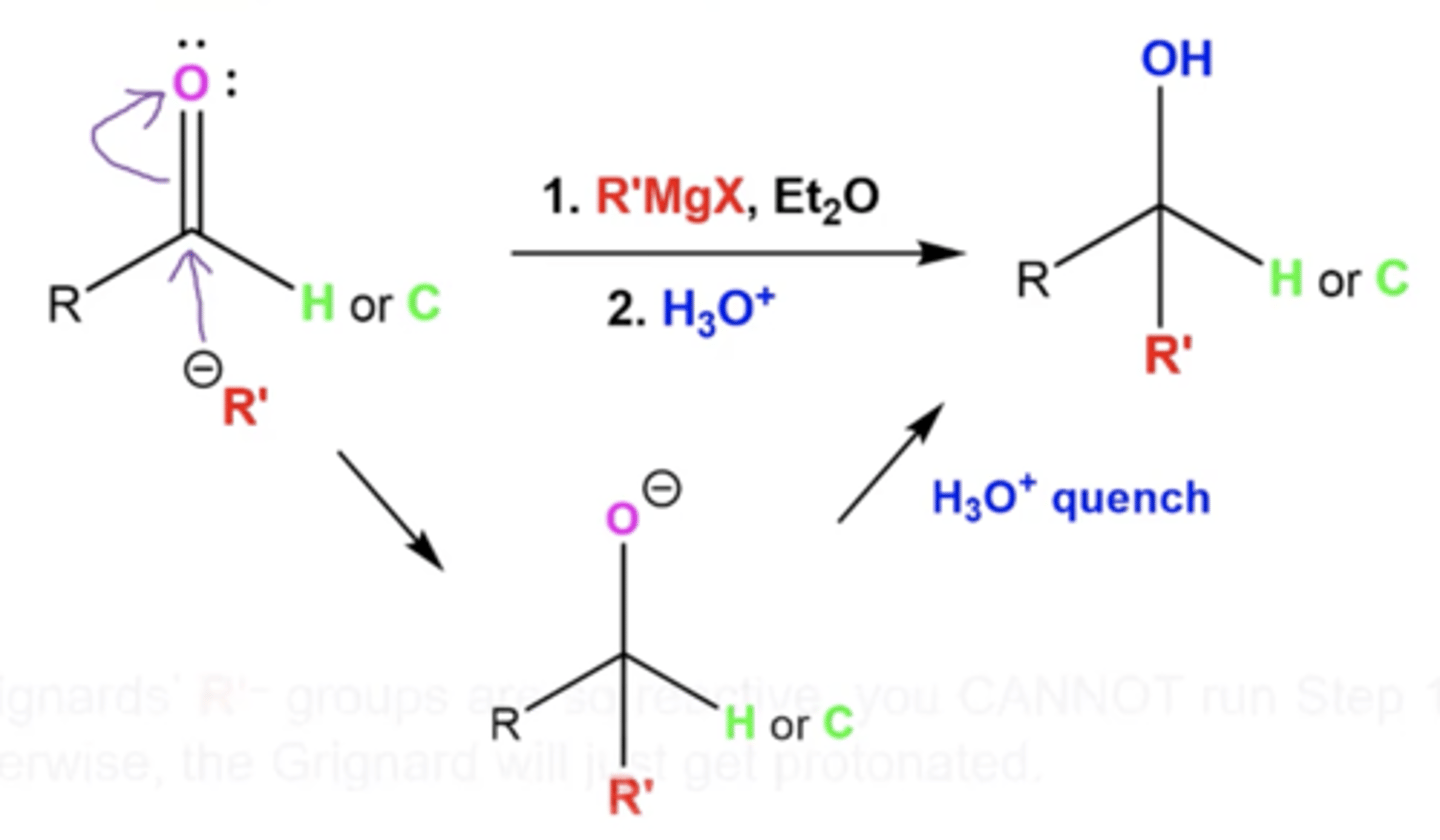
why can't you use a Grignard reagent with a protic solvent like EtOH?
EtOH will just protonate the Grignard reagent because Grignards are so reactive
what happens if you react aldehydes/ketones with these reagents:
1. R'Li, Et2O
2. H3O+
same as Grignard:
you add the R' from the Grignard and turn the carbonyl oxygen into an OH
-aldehyde = secondary alcohol
-ketone = tertiary alcohol
(the mechanism is also the same)
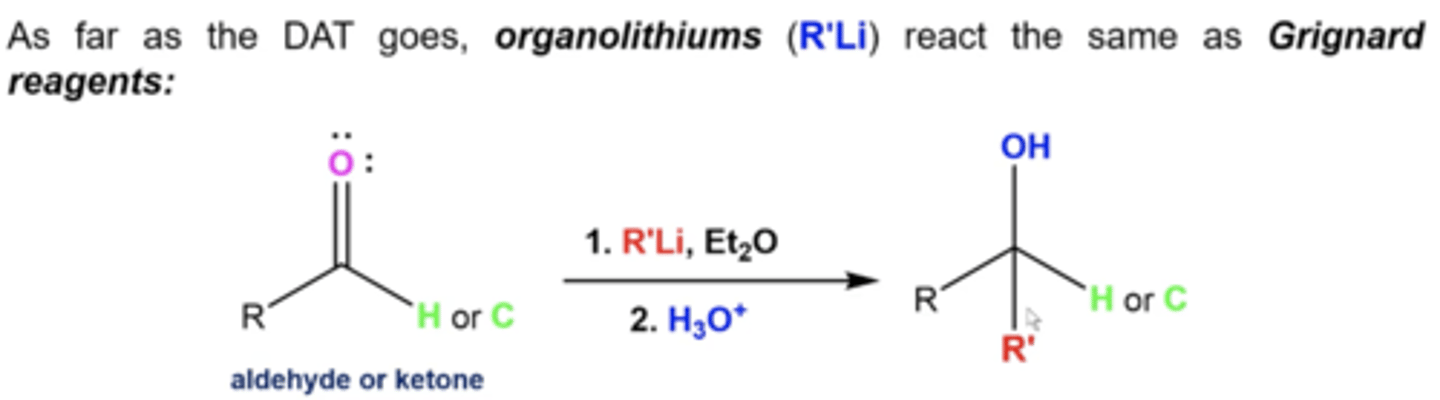
why can't you use an organolithium reagent with a protic solvent like EtOH?
EtOH will just protonate the reagent because organolithiums are so reactive
in general, what does the Wittig reaction do?
-what do Wittig reagents look like?
turns C=O into a C=C
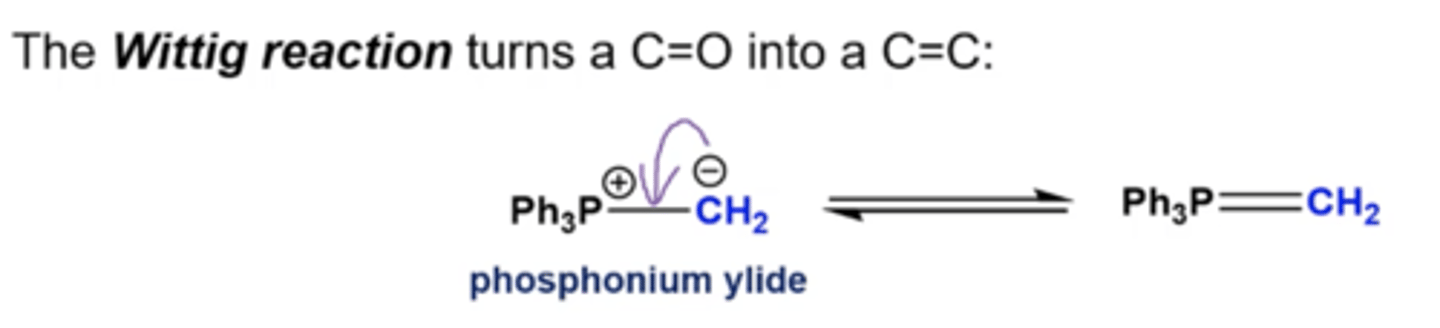
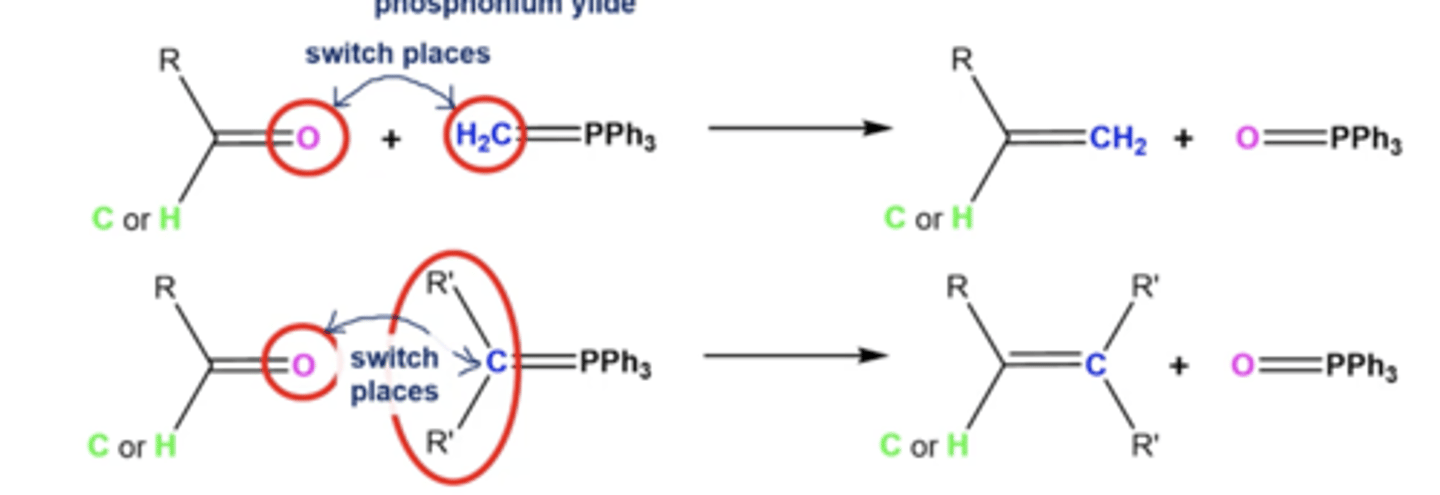
what happens if you react aldehydes/ketones with Wittig reagents?
the R' from the Wittig and the O from the ketone/aldehyde switch places
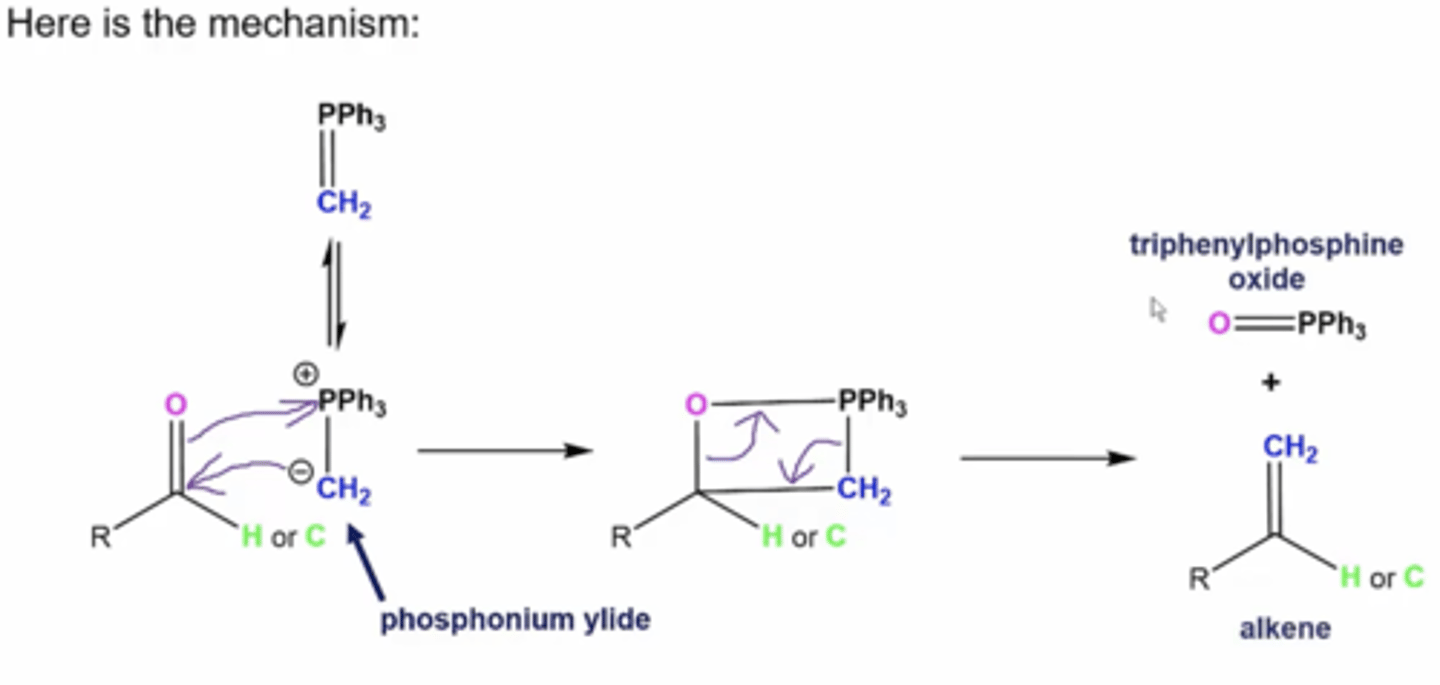
what is the mechanism for aldehydes/ketones reacting with wittig reagents?
1. -CH2 from the Wittig attacks the carbonyl carbon, which causes the oxygen to attack PPh3, forming a boxy intermediate
2. electrons swing down from oxygen and PPh3 to form our products
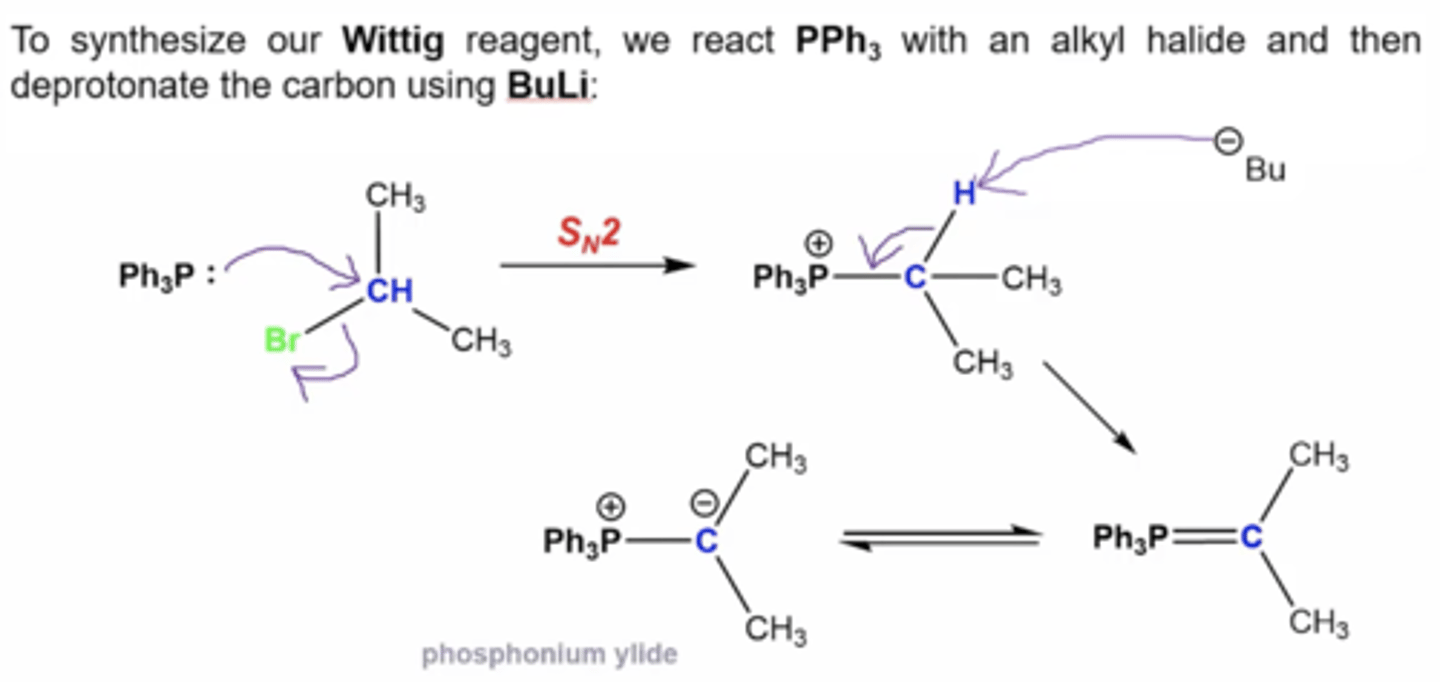
how do you synthesize the Wittig reagent?
1. react PPh3 with an alkyl halide
2. deprotonate the carbon using BuLi
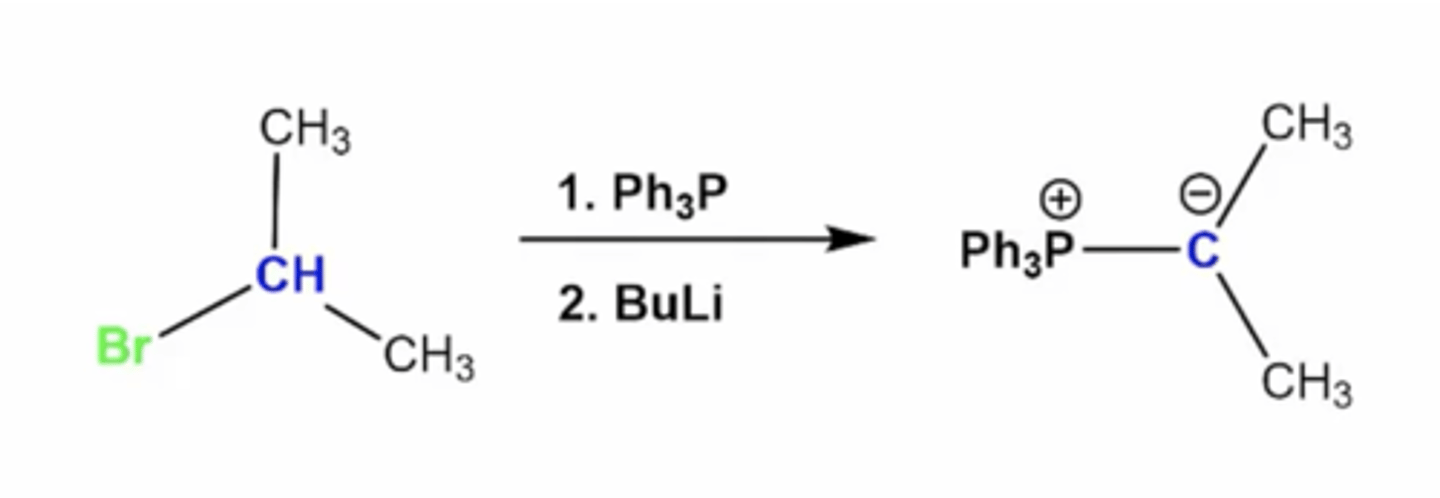
what's the mechanism to form a wittig reagent?
1. Ph3P attacks the alkyl halide carbon, kicking off the halogen via Sn2 mechanism
2. -Bu from BuLi grabs the H from the halide carbon, deprotonating it
what is it called when you have a Nucleophile attack the exterior C=C carbon that is conjugated to a C=O?
1,4-addition
or
conjugate addition
or
Michael addition
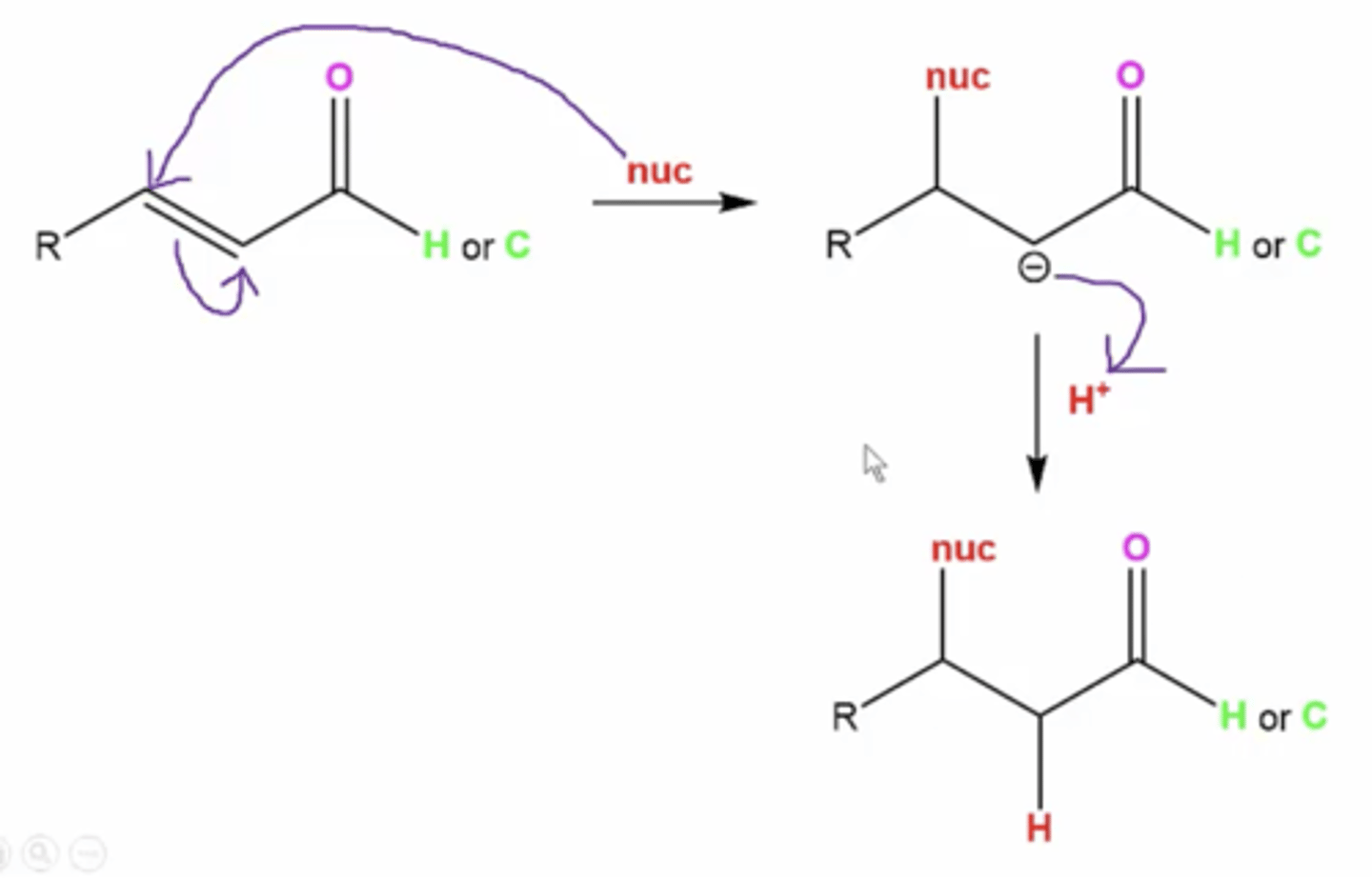
what happens when you have an aldehyde/ketone with a conjugated double bond somewhere else and react it to these reagents:
1. Nucleophile
2. H+
1. the nucleophile attacks the beta carbon
2. quench protonates the remaining carbocation
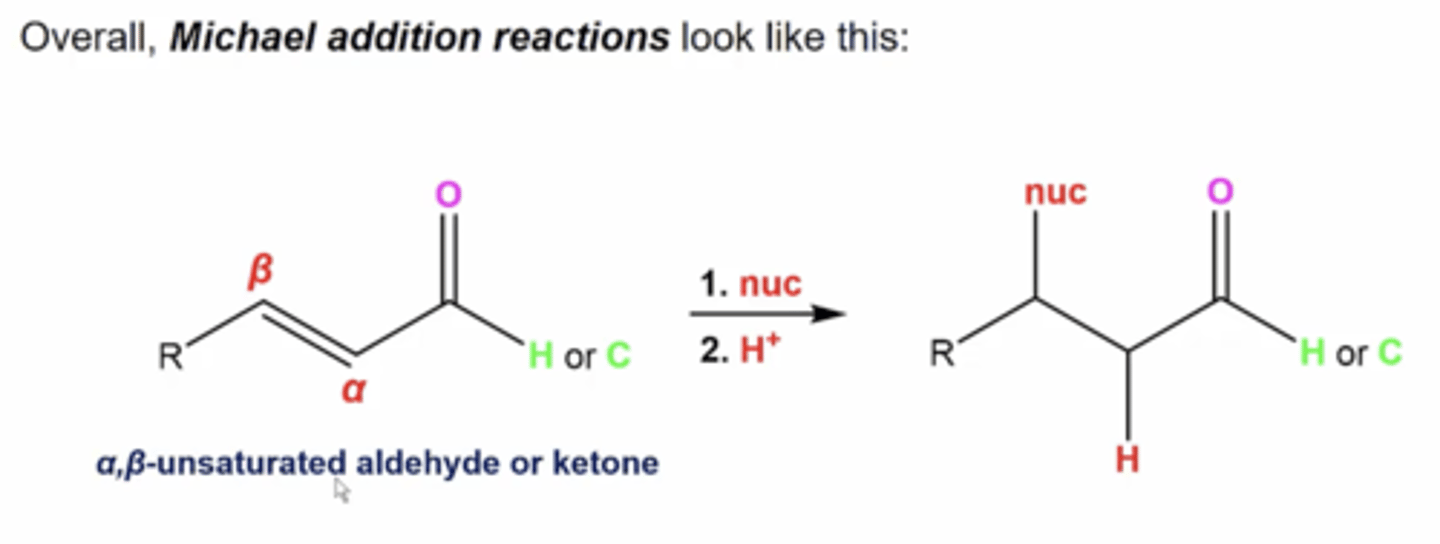
what kinds of nucleophiles prefer Michael addition over direct addition?
typically weaker nucleophiles:
-C≡N
HNR2
R2CuLi
HSR
anhydride with deprotonated hydrogen
what kinds of nucleophiles prefer direct addition over Michael addition?
(attack the carbonyl carbon = direct addition)
H- and grignards:
NaBH4 (H- source)
LiAlH4 (H- source)
RMgX (Grignard)