Bonding and Representing Molecules – Practice Questions
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Which of the following pairs of atoms are likely to form an ionic bond?
I. potassium and oxygen II. carbon and sulfur III. iron and fluorine
I and III
Which one of the following bonds would be expected to be the most polar?
a. B – I b. C – Br c. P – S d. O – S
d. O – S
Which one of the following bonds would be expected to be the least polar?
a. S – Se b. S – Cl c. N – O d. C – F
a. S – Se
In which of the following bond representations is/are the bond dipole(s) accurately represented?
II and III

Which one of the following correctly represents the bond dipole between carbon and oxygen?
B

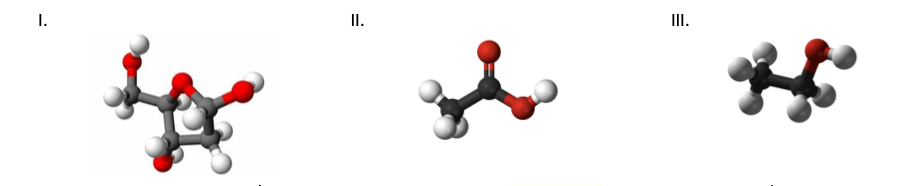
Shown below are representations of three different substances, where dark grey represents carbon, light grey/white
represents hydrogen, and red represents oxygen. The images depict the molecular formulas, which shows the actual number
of atoms in the molecule. For which of these substances is/are the empirical formula(s) the same as the molecular formula(s)?
I and III
In which one of the following substances is the central atom an exception to the octet rule?
a. SiCl4 b. BH3 c. CO2 d. NO2
+
BH3
In which one of the following substances must the central atom expand its valence shell beyond 8 electrons?
a. PCl3 b. ICl3 c. NH3 d. (CO3 )^2-
ICl3
How many total valence electrons must be represented in the Lewis structure for (SO4)^2- ?
32
Shown to the right is an incorrect Lewis structure for SO2. Identify the mistake with the structure.
a. Not enough valence e-
s
b. Incomplete octet on sulfur
c. Wrong central atom
d. Formal charge not minimized
d
Shown to the right is an incorrect Lewis structure for OF2. Identify the mistake with the structure.
a. Not enough valence e-s
b. Incomplete octet on oxygen
c. Wrong central atom
d. Incomplete octet on fluorine
a
Which one of the following is the best Lewis structure for the ClO2
- ion, including minimized formal charges?
(ignore the possible existence of resonance structures)
d
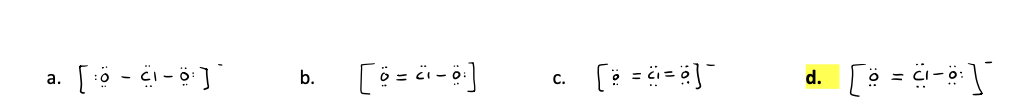
13. Which one of the following is the best Lewis structure for N2O, including minimized formal charges?
b

Which one of the following is the best Lewis structure for CSF2, including minimized formal charges?
a
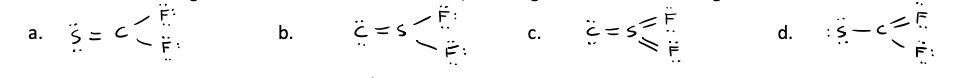
Molecules of which of the following substances is/are likely to exist as a resonance hybrid?
I. NO3
- II. O3 III. SBr2
a. I only b. II and III c. I and II d. I, II and III
c
. How many lone e- pairs must be added to the skeletal structure shown to the right complete the Lewis structure?
a. 0 b. 2 c. 4 d. 6
2
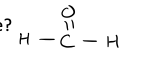
17. Draw Lewis structures for the following compounds, minimizing formal charge. Include resonance structures if applicable.
PF5 BrF2
- NH4
+
KrOCl4 NO2
- SCN-
SF4 H2O NO2
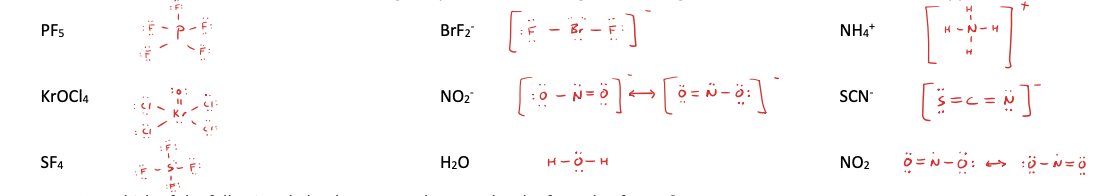
Which of the following skeletal structures has a molecular formula of C4H10?
I. II. III.
a. I only b. II only c. I and III d. I, II and III
c

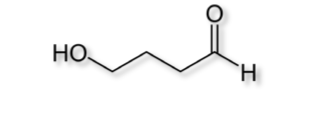
What is the name of the compound shown to the right?
a. butanoic acid b. propanoic acid c. butanal d. butanol
a
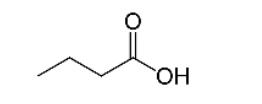
What is the name of the compound shown to the right?
a. ethanoic acid b. methanal c. ethanol d. methylamine
c
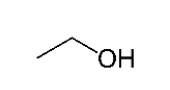
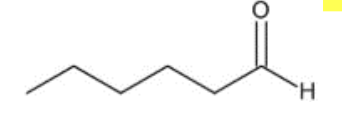
What is the name of the compound shown to the right?
a. hexanoic acid b. hexanal c. pentanoic acid d. hexylamine
b
Which of the following formula/name combination for alkanes is/are correct?
I. C3H8 / propane II. C6H12 / hexane III. CH4 / ethane IV. C4H10 / butane
a. I and III b. II and III c. I and IV d. I, II, and IV
C
Identify all functional groups present in the molecule.
alcohol
aldehyde
amine
carboxylic acid
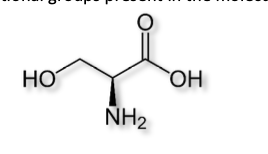
Identify all functional groups present in the molecule.
alcohol
aldehyde
amine
carboxylic acid