chapter 19 - AP2
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
78 Terms
overview of blood
About 5 liters of the fluid connective tissue blood—8% of the total body weight—circulate through the heart and blood vessels at all times.
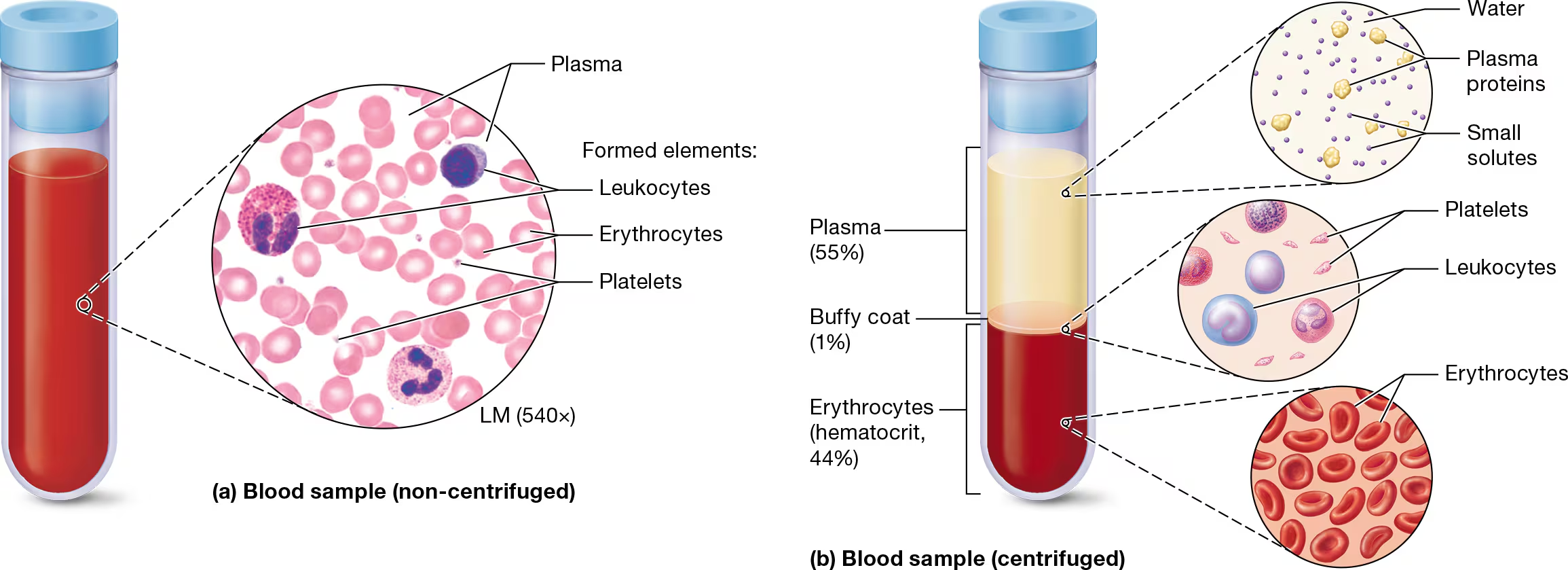
What are the visible elements of a blood sample
plasma, the liquid extracellular matrix,
formed elements
what are the three formed elements in blood?
erythrocytes, or red blood cells (RBCs);
leukocytes, or white blood cells (WBCs)
tiny cellular fragments called platelets
three distinct layers - what are they and what percent do they make up of a blood sample?
when a blood sample is placed in a centrifuge and spun rapidly, it forms three layers
plasma coat - 55% - water, plasma proteins, small solutes
Buffy coat - 1% - leukocytes and platelets
Erythrocytes (Hematocrit, 44%) - hematocrit is the percentrage of blood composed of erythrocytes
what are the functions of blood?
gas exchange- oxygen is transported to the tissues from the lungs and CO2 is transported from the tissues to the lungs
solute distribution - Plasma transports many solutes, including nutrients, hormones, and wastes. Additionally, blood transports ions and plays a role in regulating ion concentrations in the tissues.
performing immune functions - leukocytes and immune proteins use blood as a transport system
maintianing body temperature - blood carries away heat from the metabolically active tissues, keeping constant temperature in tissues with a high rate of catabolism
sealing damaged blood vessels by forming clots - When a blood vessel is broken, platelets and certain proteins form a blood clot that seals the damaged vessel, preventing excessive blood loss
preserving acid - base homeostasis - The pH remains fairly constant (7.35-7.45) because blood composition controls many of the body’s most important buffer systems. Also, many plasma proteins act as buffers.
stabalizing blood pressure - blood volume is a factor of blood pressure
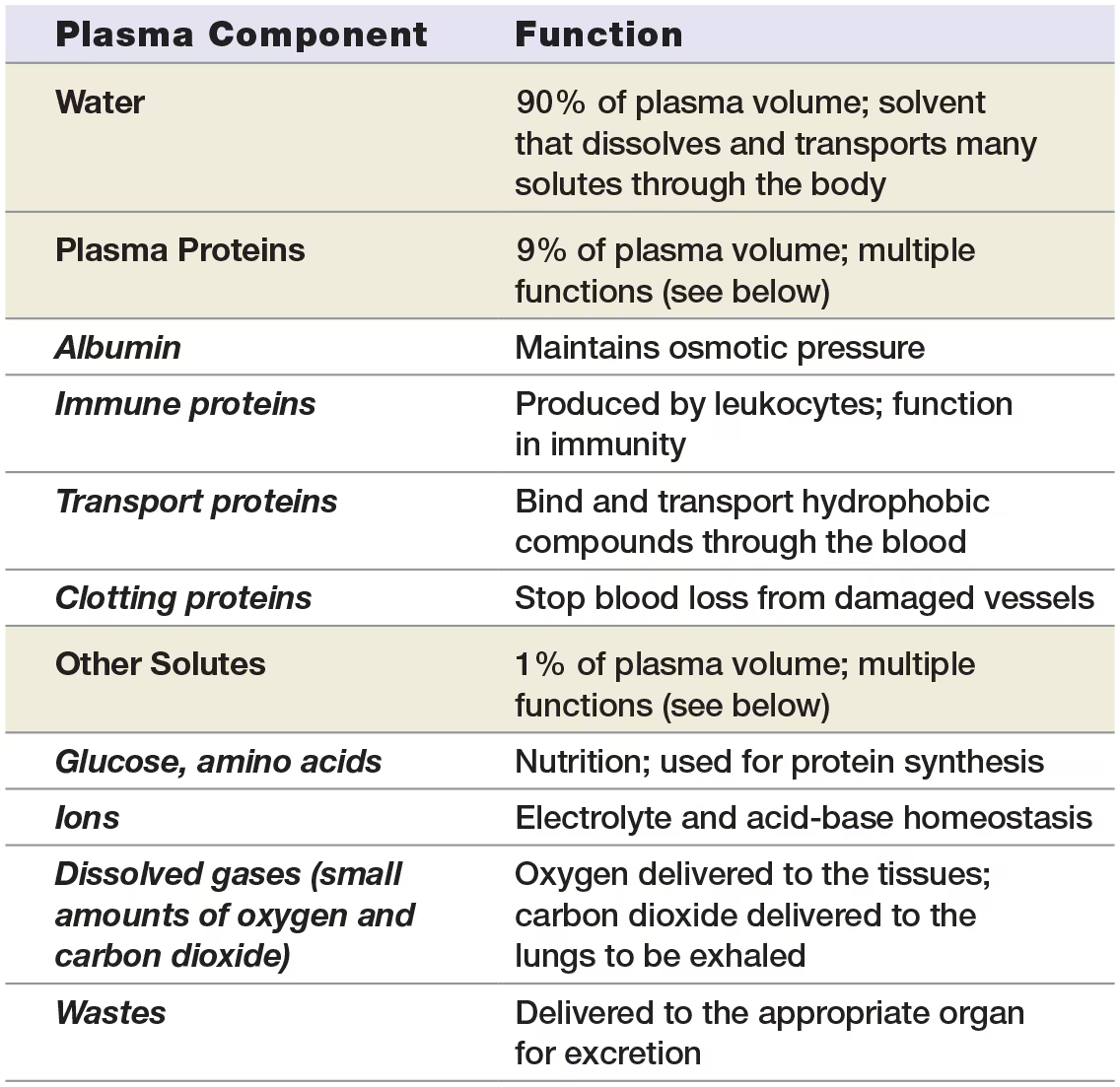
Plasma
consists primarily of water - about 90%
the amount of water in the plasma determines its viscosity, bloodflow becomes sluggish if there is less water than needed
Plasma proteins - 9% of plasma
made mostly by the liver
too large to fully dissolve in the water portion of the plasma, so they form a colloid
important plasma proteins
Albumin - responisble for blood’s colloid osmotic pressure
y-globulins (antibodies) - made by leukocytes
Transport proteins - hydrophobic compunds need to be bound to transport proteins to safely travel through the blood instead of forming in clumps (globular proteins called α- and β-globulins and lipoproteins)
Clotting proteins - A blood clot, which is a collection of platelets and clotting proteins, stops bleeding from an injured blood vessel.
Remaining 1% of plasma
solutes such as nutrients like glucose, amino acides, nitrogenous wastes, ions, small amounts of dissolved oxygen and carbon dioxide
Readily exchanged between the blood and the interstitial fluid in most capillary beds
Erythrocytes and hematocrit
hematocrit is typically higher in males than females (40-50% vs 36-44%) due to males larger body size, testosterone, and greater amounts of erythropoietin
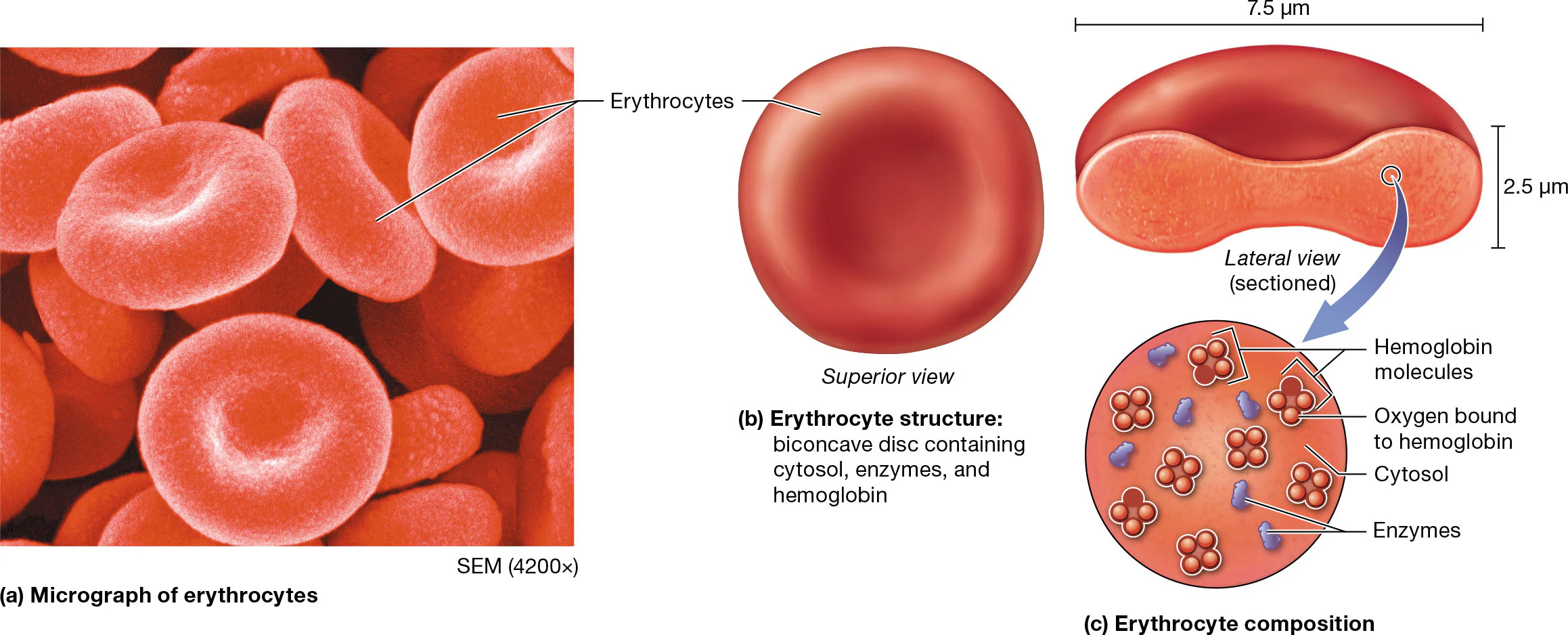
Erythrocyte structure
typically a biconcave disc, giving it a large surface-to-volume ratio. this is critical to their role in gas exchange
mature erythrocytes are anucleate and lack most other organelles, including mitochondria
this means that these cells cannot perform oxidative catabolism or protein synthesis.
contain about a billion molecules of hemaglobin
adult males have more hemaglobin per red blood cell than women caused mostly by testosterone and erythropoietin
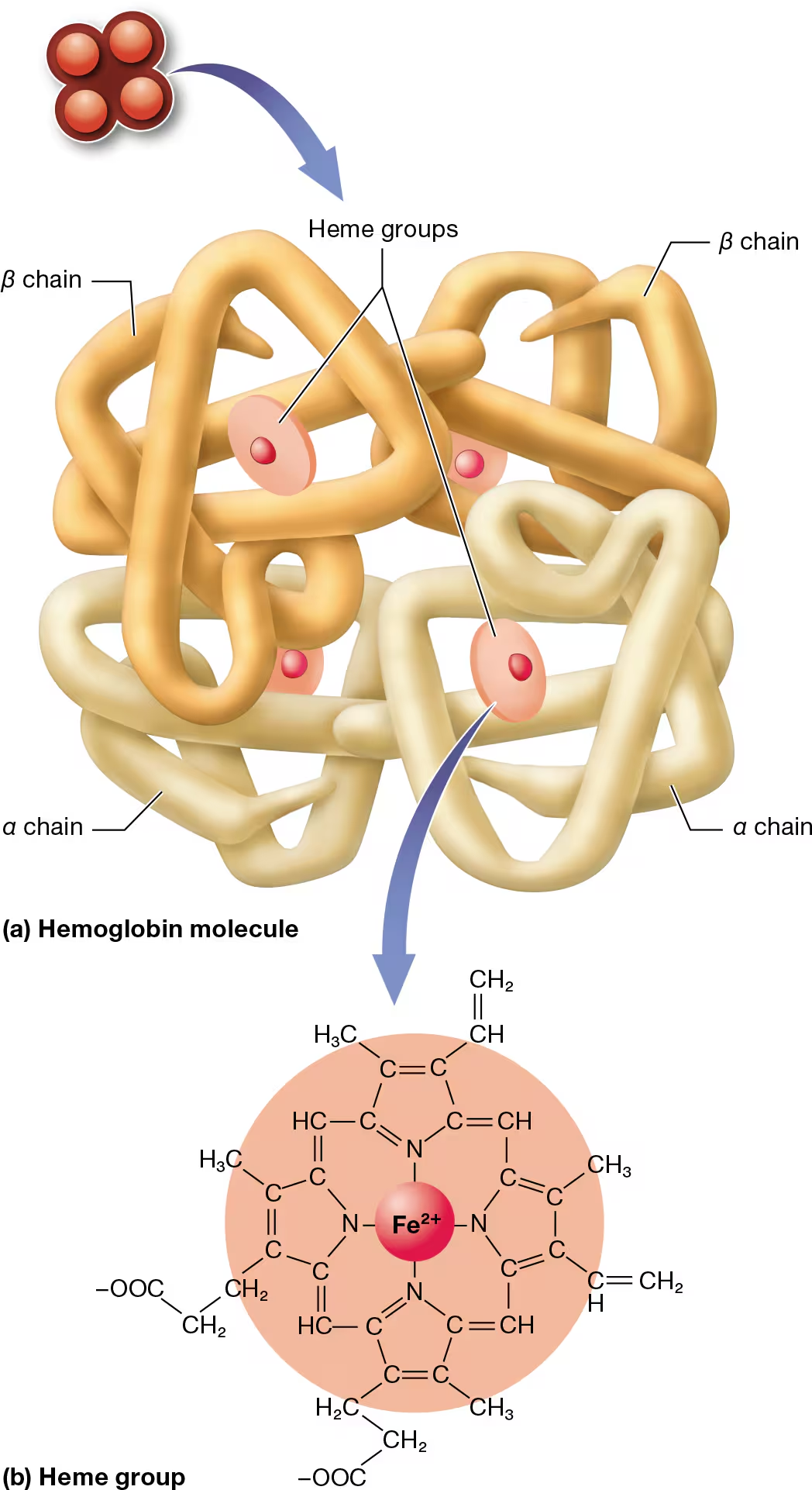
hemaglobin structure
large protein consisting of 4 polypeptide units
2 alpha and 2 beta chains
each polypeptide is bound to an iron-containing compound (heme group)
heme binds to oxygen in parts of the body where oxygen is high (like the lungs)
the bound heme groups make up oxyhemaglobin
when oxygen levels are low, hemaglobin relaxed and lets go of oxygen, becoming deoxyhemaglobin
when hemaglobin reacts with oxygen, it produces a red color
the shade of red is determined by how much oxygen is bound to the hemaglobin
bright red = all 4 heme groups are oxygenated (this is often the case in systematic arteries)
dark red/purple color = more of the hemoglobin is deoxyhemoglobin = often in systemic veins
In venous blood where the oxygen level is low, hemoglobin binds to carbon dioxide (CO2), to form carbaminohemoglobin.
Approximately 23% of the carbon dioxide in blood is transported in this manner.
Hemoglobin also binds to carbon monoxide (CO), which forms carboxyhemoglobin.
Unfortunately, carbon monoxide binds to hemoglobin more strongly than does oxygen and has an affinity for hemoglobin about 200 times greater than that of oxygen. Furthermore, CO changes the shape of hemoglobin so that the oxygen already bound to hemoglobin cannot be released into the tissues. This makes exposure to a high level of carbon monoxide lethal—it severely decreases the oxygenation of body tissues.
(Contrary to popular perception, systemic venous blood is not blue. Rather, veins simply look blue because of an optical illusion related to light refraction.)
Erythrocytes lack the cellular machinery to repair this damage, so their average lifespan is only 100–120 days, and the body must continuously make new erythrocytes.
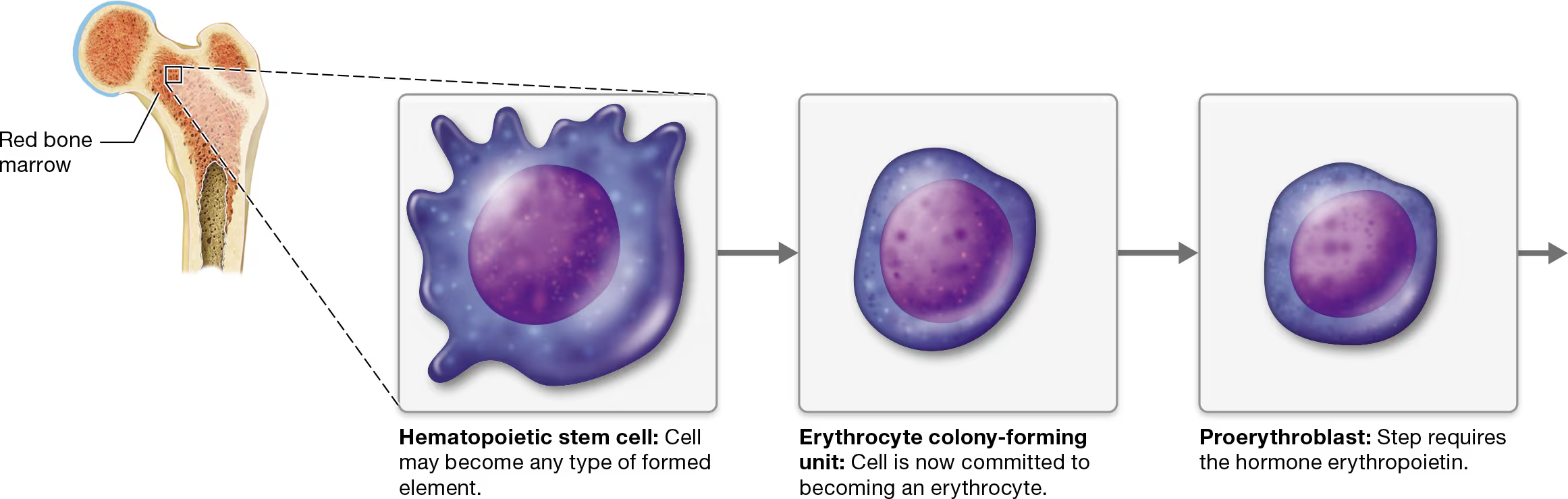
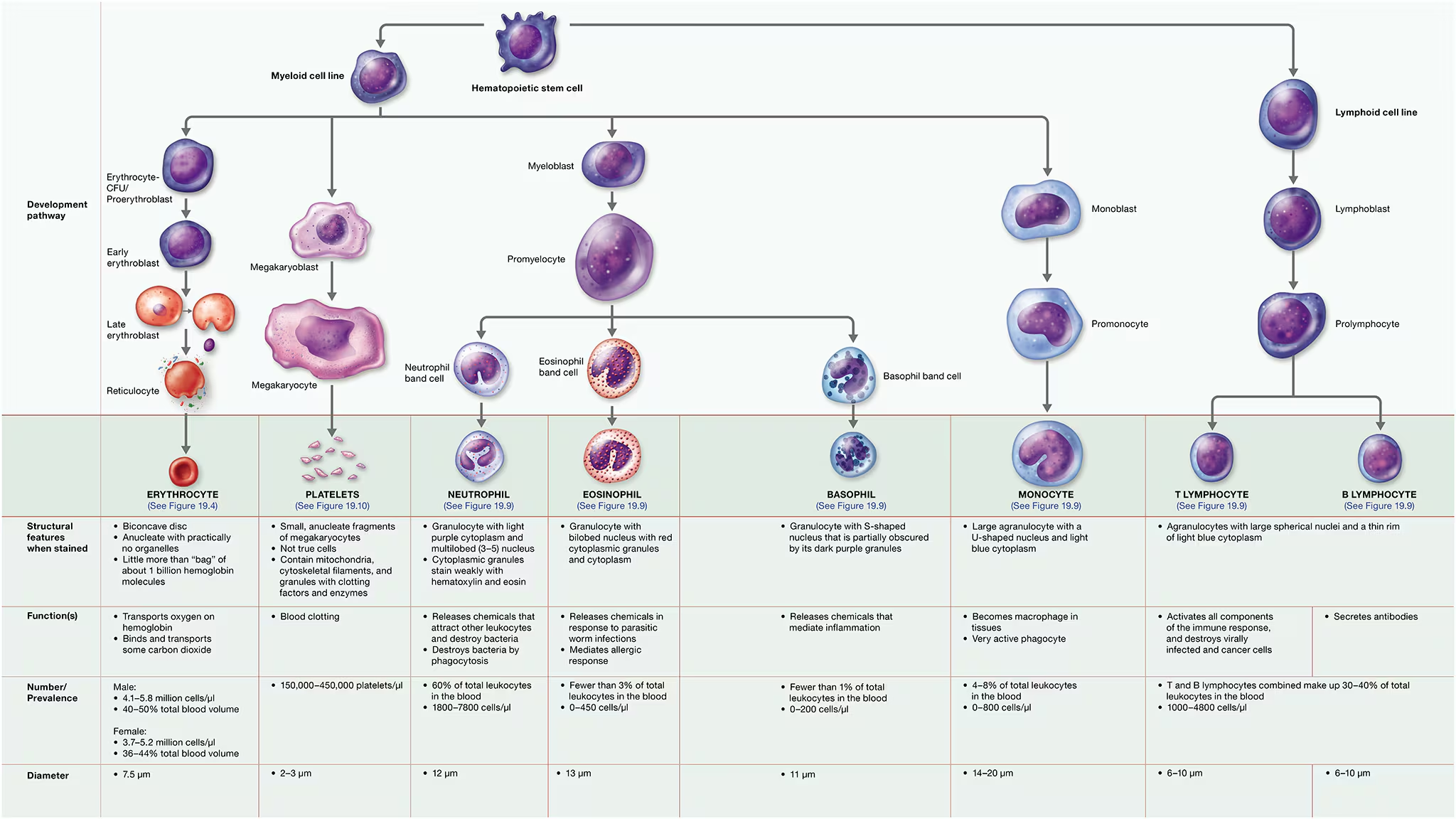
hematopoiesis - The process of differentiation and maturation of the formed elements of blood.
Erythropoiesis (ih-rith′-roh-poy-EE-sis), the formation of erythrocytes, is part of the larger process of hematopoiesis
250 billion RBCs per day are made
Hematopoietic stem cell (HSC)
Erythrocyte colony-forming (CFU) - commitment point
CFUs differenciate into proerythroblasts - requires the presence of erythropoietin
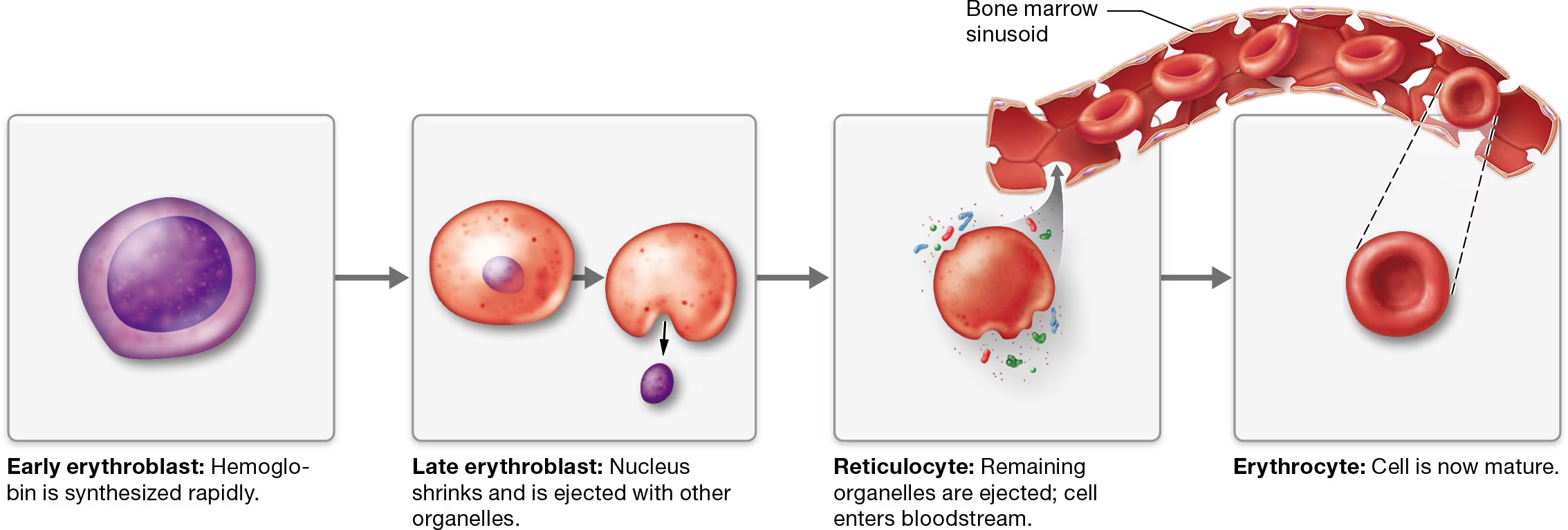
they develope into erythroblasts - rapidy synthesize hemaglobin and other proteins, as they mature, their nuclei shrink and are eventually ejected from the cells, at which point they are called reticulocytes
These cells (reticulocytes) retain some organelles, particularly ribosomes.
When they eject these remaining organelles by exocytosis, they enter the bloodstream by squeezing through the large pores in the sinusoidal capillaries of the bone marrow and are considered erythrocytes. The entire process takes 5–7 days.
if the number of RBCs in the bloodstream is inadequate, then reticulocytes are released in small number because they are able to bind to oxygen wit htheir hemaglobin
Indeed, an elevated reticulocyte count is a sign of slow blood loss, as occurs with a stomach ulcer.
Regulation of erythropoiesis
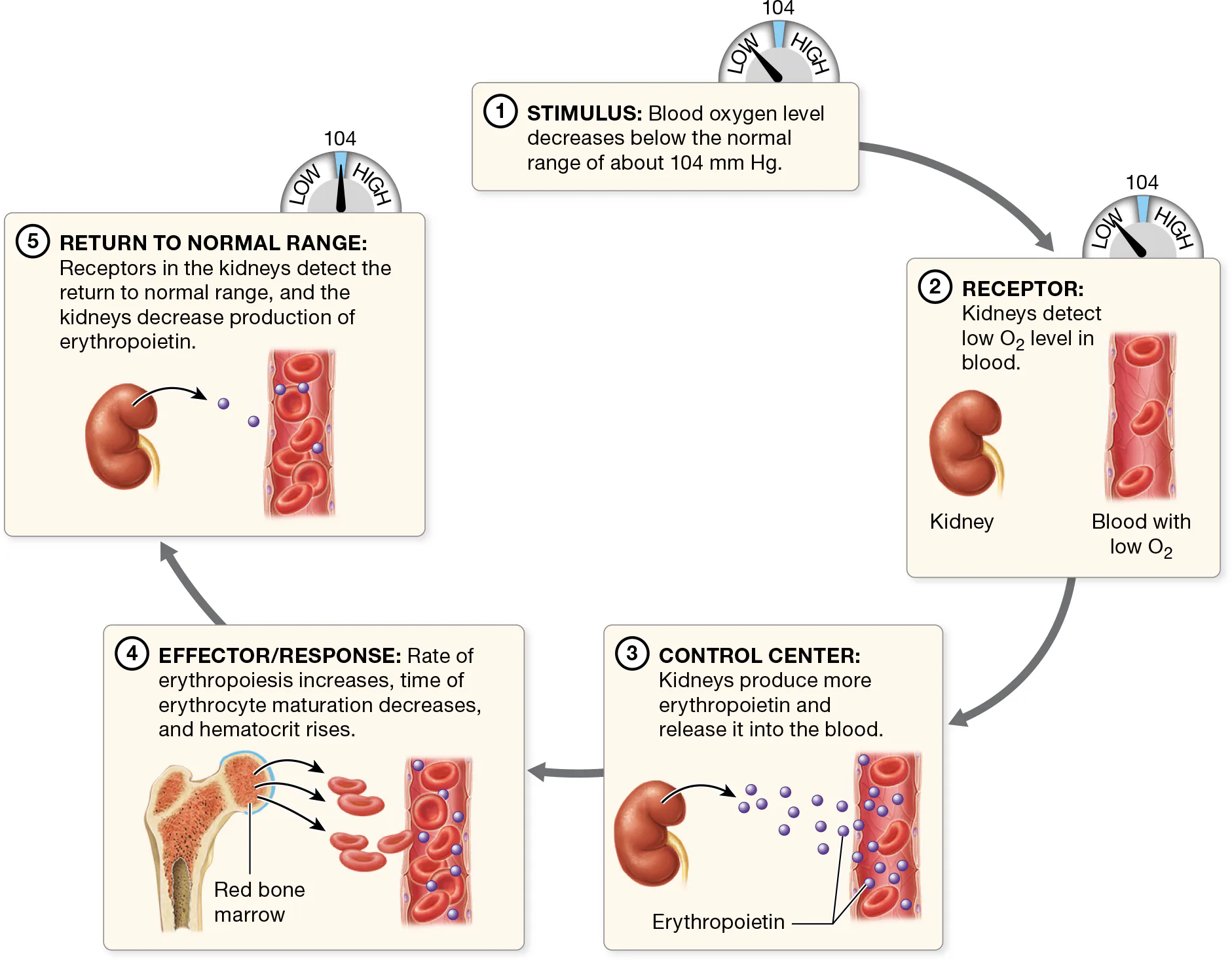
regulation of hematocrit by negative feedback loop, if too low, not enough oxygen to tissues. If too high, the blood becomes thicker (increased viscocity) making bloodflow sluggish.
Stimulus: Blood oxygen level decreases below the normal range of about
104 mm Hg. A decrease in the oxygen content of blood is usually due to respiratory problems, but it may also result from heart conditions or reduced availability of oxygen
Receptor: Kidney cells detect a low oxygen level in blood.
Specialized cells in the kidneys act as chemoreceptors and monitor the blood’s oxygen content.
Control Center: Kidneys produce more erythropoietin and release it into the blood.
(which communicates with hematopoietic stem cells (HSCs) in the bone marrow.)
Effector/Response: Rate of erythropoiesis increases, time of erythrocyte maturation decreases, and hematocrit rises.
Erythropoietin can also trigger the replacement of yellow bone marrow with red bone marrow
Return to Normal Range: Receptors in the kidneys detect the return of blood oxygen to the normal range, and the kidneys decrease production of erythropoietin.
how can growth factors increase hematocrit?
In addition to erythropoietin, chemicals known as growth factors can increase the production of erythrocytes.
Growth factors are produced by cell types such as endothelial cells in blood vessels and fibroblasts in connective tissue.
They aid in the transition from yellow bone marrow to red bone marrow, and also trigger mitosis of hematopoietic stem cells.
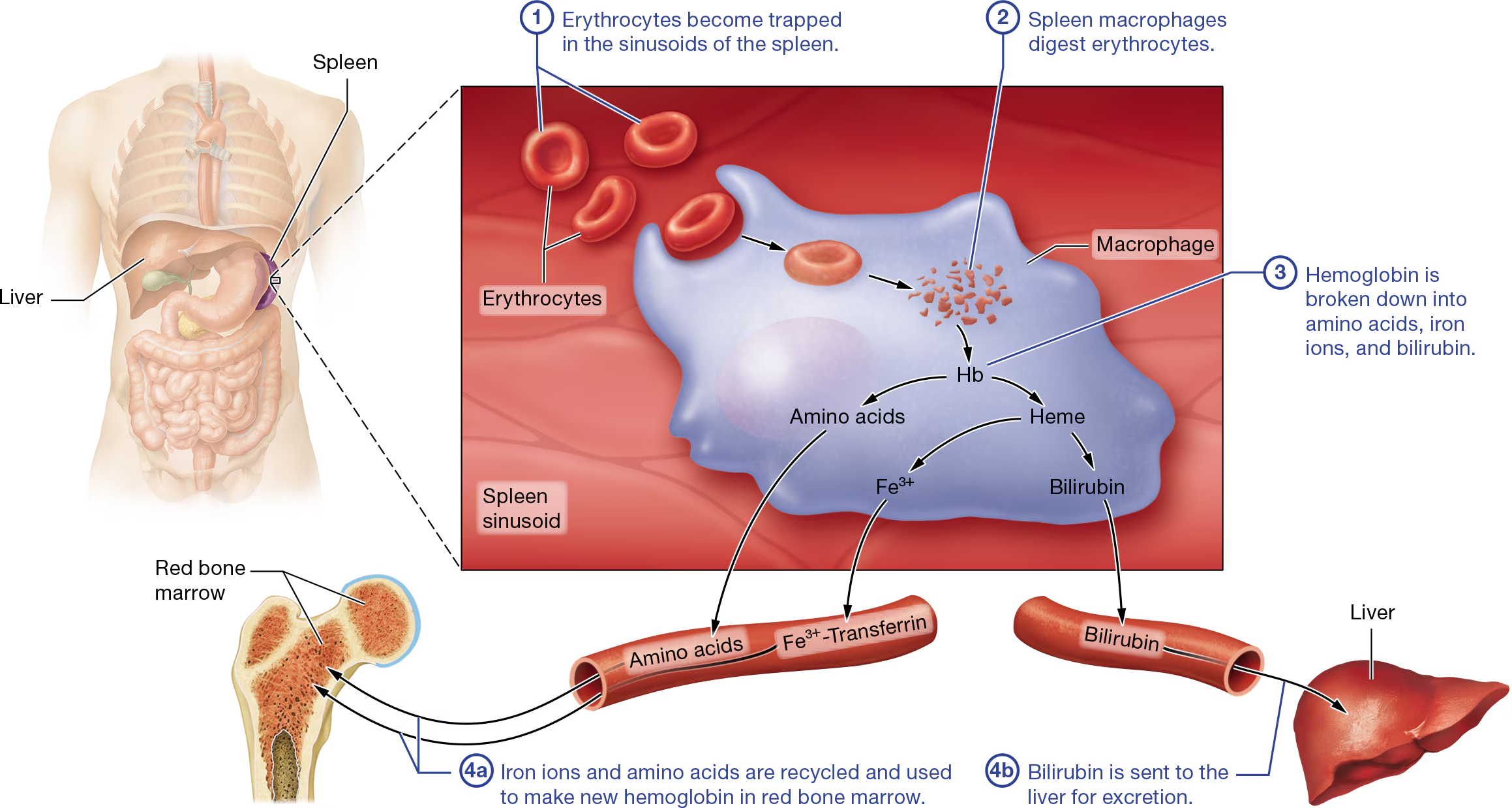
Erythrocyte Death
STEP 1:Erythrocytes become trapped in the sinusoids of the spleen. Older erythrocytes are not flexible enough to exit the tortuous sinusoids.
STEP 2:Spleen macrophages digest erythrocytes. In the sinusoids, erythrocytes encounter leukocytes called macrophages
STEP 3:Hemoglobin is broken down into amino acids, iron ions, and bilirubin.
the remainder of the heme is converted first into the waste product biliverdin, a greenish pigment (verd-=“green”).
Generally, biliverdin is then converted into a second waste product, the yellowish pigment bilirubin (bil-ih-ROO-bin).
Interestingly, biliverdin is responsible for the greenish color you see in bruises. As the biliverdin is converted to bilirubin, the bruise becomes yellow.
STEP 4a: Iron ions and amino acids are recycled and used to make new hemoglobin in red bone marrow. You can see in Figure 19.6 that the iron ions are transferred to a protein called transferrin (trans-FER-in) that carries them through the blood.
STEP 4b: Bilirubin is sent to the liver for excretion. At the same time the events in ④a are occurring, bilirubin enters the blood and is transported to the liver, where it is modified and excreted in feces and urine.
Anemia
decreased oxygen-carrying capacity of the blood.
Symptoms:
pallor (plan skin, gums, and/or nail beds)
fatigue
weakness
shortness of breath
many types of anemia increase the number of reticulocytes in the blood as the body boosts erythrocyte production.
rapid heart rate, which is the body’s attempt to compensate for impaired oxygenation of the tissues by increasing cardiac output.
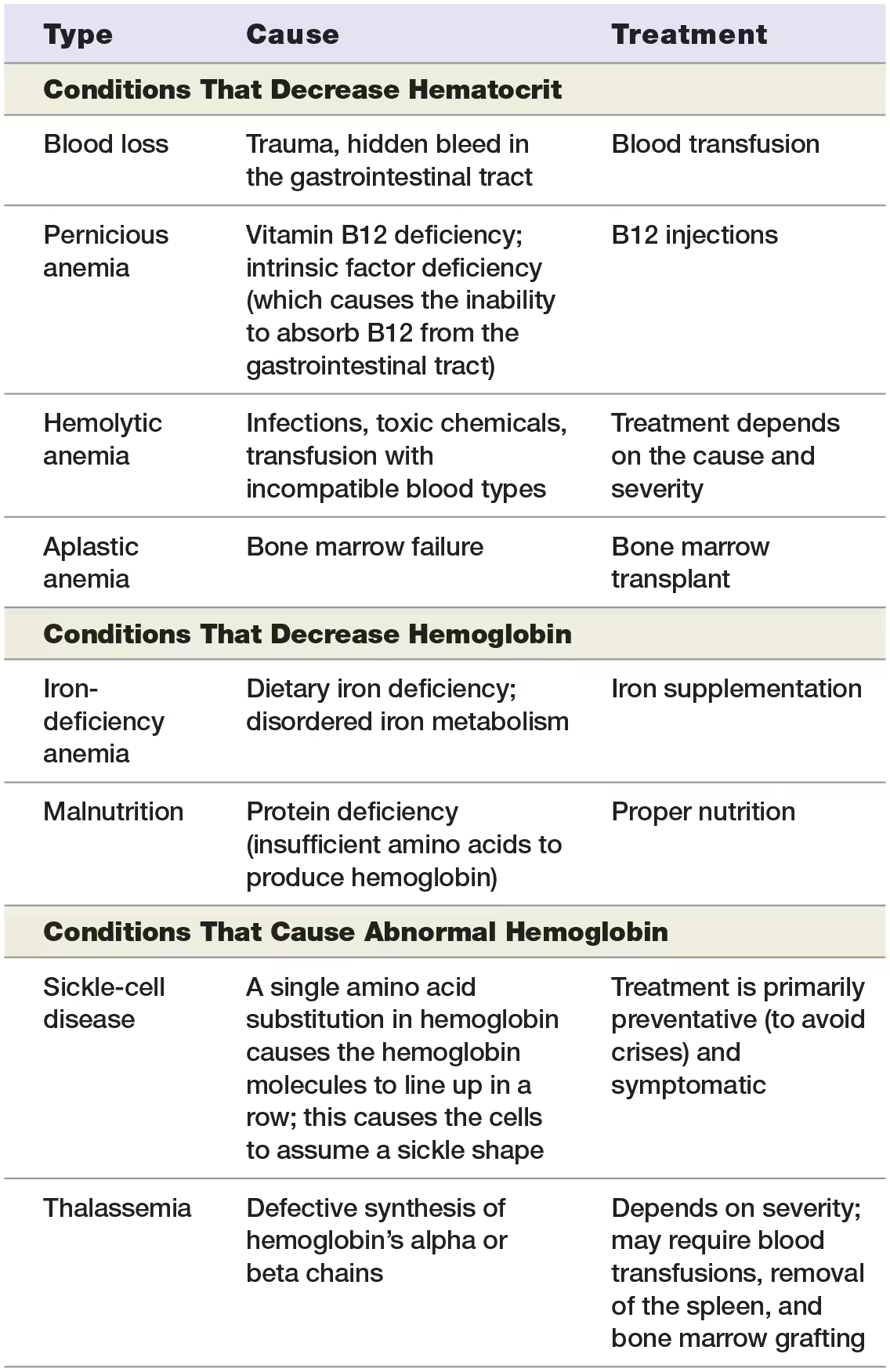
what is the most common type of anemia?
iron-deficiency anemia
due to inadequate dietary iron, reduced intestinal absorption of dietary iron, or slow blood loss (including menstruation).
Without functional iron-containing heme groups, erythroblasts cannot make hemoglobin.
Erythrocytes in an individual with iron-deficiency anemia are generally small and pale, and the number of erythrocytes and hematocrit may actually be normal or even slightly elevated.
Anemia chronic disease
such as in cancer, which can produce chemicals the interfere with iron transport to the red bone marrow from the liver
Decreased hemoglobin may also be due to malnutrition, vitamin B6 deficiency, certain drugs, poisoning with heavy metals such as lead, or even pregnancy.
Decreased Hematocrit
factors that cause decreased hematocrit numbers:
acute or chronic blood loss
Pernicious anemia results from vitamin B12 deficiency, which interferes with DNA synthesis of rapidly dividing cells, including hematopoietic cells in bone marrow.
Erythrocyte destruction can lead to hemolytic anemia.
Causes of hemolytic anemia include bacterial infections, diseases of the immune system or liver, and lead poisoning.
Finally, the red bone marrow may stop producing erythrocytes, which results in aplastic anemia
Certain medications or exposure to ionizing radiation may induce aplastic anemia, but the cause in many cases is unknown.
Abnormal hemaglobin
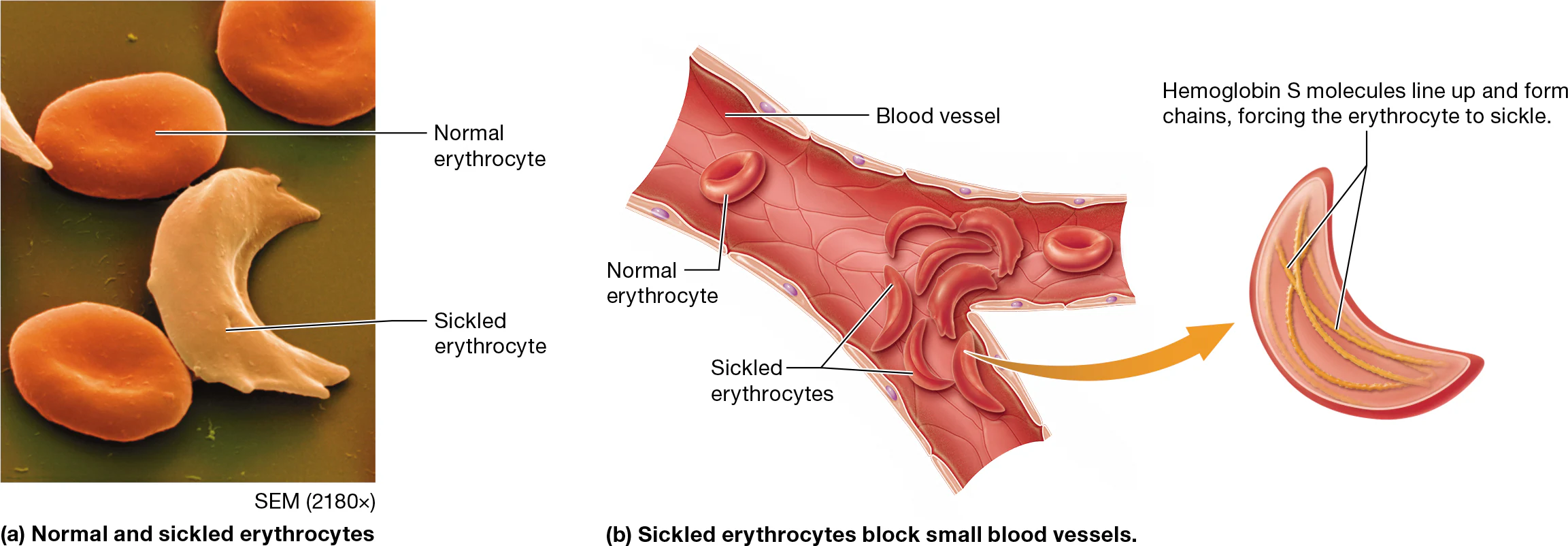
the most common condition from abnormal hemaglobin is sickle-cell disease
Individuals with two copies of the defective gene have sickle-cell disease, in which they produce an abnormal hemoglobin called hemoglobin S, or HbS.
When the oxygen level is low, HbS proteins line up in a row, forcing the erythrocytes into a curved “sickle” shape (Figure 19.7).
The sickle-shaped cells get stuck in capillary beds, which leads to ischemia and tissue damage.
The sickled cells are eventually destroyed; this lowers the number of circulating erythrocytes and causes anemia.
Polycythemia
when hematocrit is too high
primary polycythemia: (myeloproliferative disorder)
bone marrow produces too many erythrocytes
Most common type of primary polycythemia is polycythemia vera - a genetic condition that leads to excess erythrocytes, leukocytes, and platelets
Symptoms: enlarged spleen, headaches, itching triggered by hot water, and easy bruising. Individuals with the condition have an increased risk of developing abnormal blood clots, bone marrow failure, and/or acute leukemia
Treatment: : bloodletting. Although we no longer use bloodletting to treat everything from syphilis to menstrual pain, the removal of controlled amounts of blood via phlebotomy remains an effective therapy for polycythemia vera.
Secondary Polycythemia (more common):
caused by something external to the bone marrow
Ex of physiological polycythemia (low oxygen level in their blood triggers erythropoietin secretion from their kidneys):
the body’s response to decreased oxygen in the blood,
individuals with chronic heart or lung disease often have a higher hematocrit
people living at high altitudes, where there is a relatively low level of oxygen in the atmosphere (Athletes often train at high altitudes to induce secondary polycythemia in the hopes of improving their endurance)
Ex of non-physiological polycythemia causations:
cancer
when individuals inject testosterone or other anabolic steroids, as these increase erythrocyte production.
the hematocrit may simply appear to be elevated in individuals who are dehydrated—in such cases, the absolute number of cells is normal, but the percentage of cells relative to plasma is abnormal
Non-physiological secondary polycythemia may not need to be treated as a condition on its own, as it generally improves when its underlying cause resolves.
Leukocytes
larger than RBCs
have a prominent nuclei
generally have functions outside of the blood, but sue blood as a transport system to reach nearly all the tissues in the body, where they squeeze out of capillary beds
what aare the 2 basic varieties of leukocytes?
granulocytes
agranulocytes
what 2 dyes are cells treated with in a blood smear to examine the cell’s features?
the red, acidic dye eosin and the dark purple, basic dye hematoxylin
you classify granulocytes into three catigories based on the color of their granules after beinf stained
Granulocytes
contain specific cytoplasmic granules that the cells release when activated
easy to distiguish by their unusual nuclei
each cell actually has a single nucleus with multiple lobes that are connected by thin bands of nuclear material.
All granulocytes contain general lysosomal granules as well as specific granules unique to each type of granulocyte.
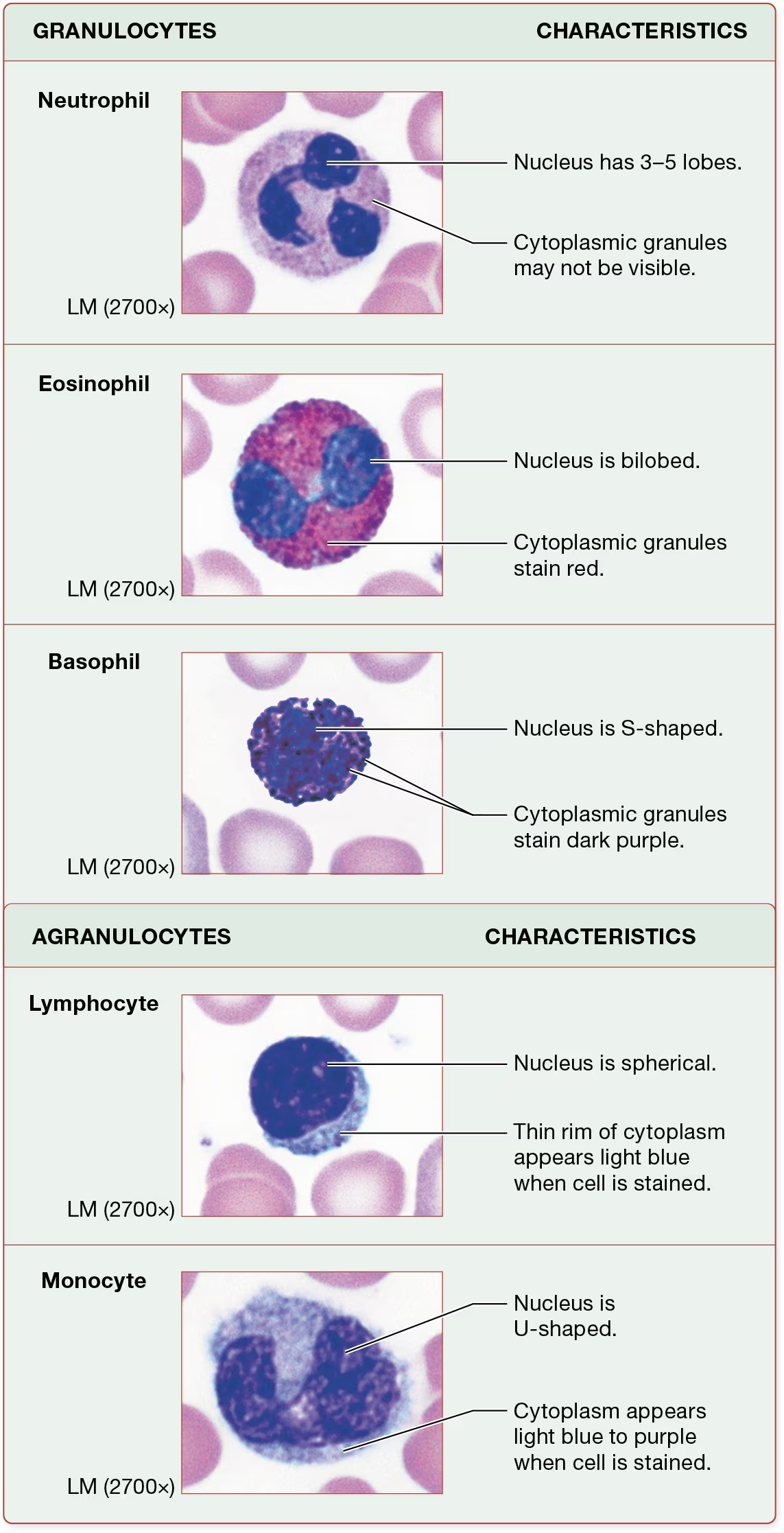
granulocytes:neutrophils
most common type of leukocyte (60%)
granules are weakly basic so they dont stain strongly with either dye
granules have a very light pink to purple color, making them not very visible against their lilac-colored cytoplasm
nuclei have 3-5 lobes that can vary in shape: the reason they are called polymorphonucleocytes (PMNs or polys)
Neutrophils are attracted to injured cells by chemicals released by the damaged cells, a process called chemotaxis
When neutrophils reach the damaged tissue, they exit the blood and release the contents of their granules.
These contents directly kill bacterial cells, enhance inflammation, and attract more neutrophils and leukocytes to the area.
Neutrophils are very active phagocytes that ingest and destroy bacterial cells.
Granulocytes: Eosinophils
relatively rare leukocytes (3% of leukocytes)
the granules of eosinophils take up the acidic dye eosin, which is because many of them have a basic pH.
their granules appear bright red.
their nuclei are bilobed, resembling a barbell
involved when the body is infected with parasitic worms an in allergic reactions
Their granules contain substances that assist in these functions, including enzymes and toxins that are specific to parasites, as well as chemicals that mediate (bring about) inflammation.
In addition, eosinophils are phagocytes and ingest foreign compounds that have been bound by proteins of the immune system.
Granulocytes; Basophils (Basic liking)
least common leukocyte (1%)
basophils contain acidic granules that take up the basic dye hematoxylin
this stains the granules dark purple-blue—they are so dark they almost completely obscure the basophils’ nuclei, which are typically S-shaped.
Basophils release chemicals from their granules that mediate inflammation.
Agranulocytes: lymphocytes
lack specific cytoplasmic granules, although like granulocytes, they do contain lysosomes.
2nd most neumerous type of leukocyte (30-34%)
contain a large and spherical nucleus and a thin rim of cytoplasm that is light blue when stained
Both B and T lymphocytes are activated by cellular markers called antigens
Antigens, which are generally glycoproteins, are present on all cells and most biological compounds.
B-lymphocytes (plasma cells)
produce antibodies that bind to antigens and remove them from tissues
each B-Lymphocyte population makes SPECIFIC antibodies that allow them to only bind to one antigen
T-Lymphocytes
also show specificity, but do not produce antibodies
populations of T lymphocytes have specific receptors for individual antigens.
When the receptors are bound, T lymphocytes activate other components of the immune system and directly destroy abnormal body cells, such as cancer cells and those that are infected with viruses.
Agranulocytes: Monocytes
lack specific cytoplasmic granules, although like granulocytes, they do contain lysosomes.
Monocytes are the largest leukocytes and are 4-8% of the total leukocytes
large U-shaped nuclei and cytoplasm that becomes light blue or purple when the cells are stained.
Monocytes remain in the blood for only a few days before they exit the capillaries and enter the tissues, where some mature into very active phagocytes called macrophages
Macrophages ingest dead and dying cells, bacteria, antigens, and other cellular debris.
In addition, they activate other parts of the immune system by displaying phagocytosed antigens to other leukocytes.
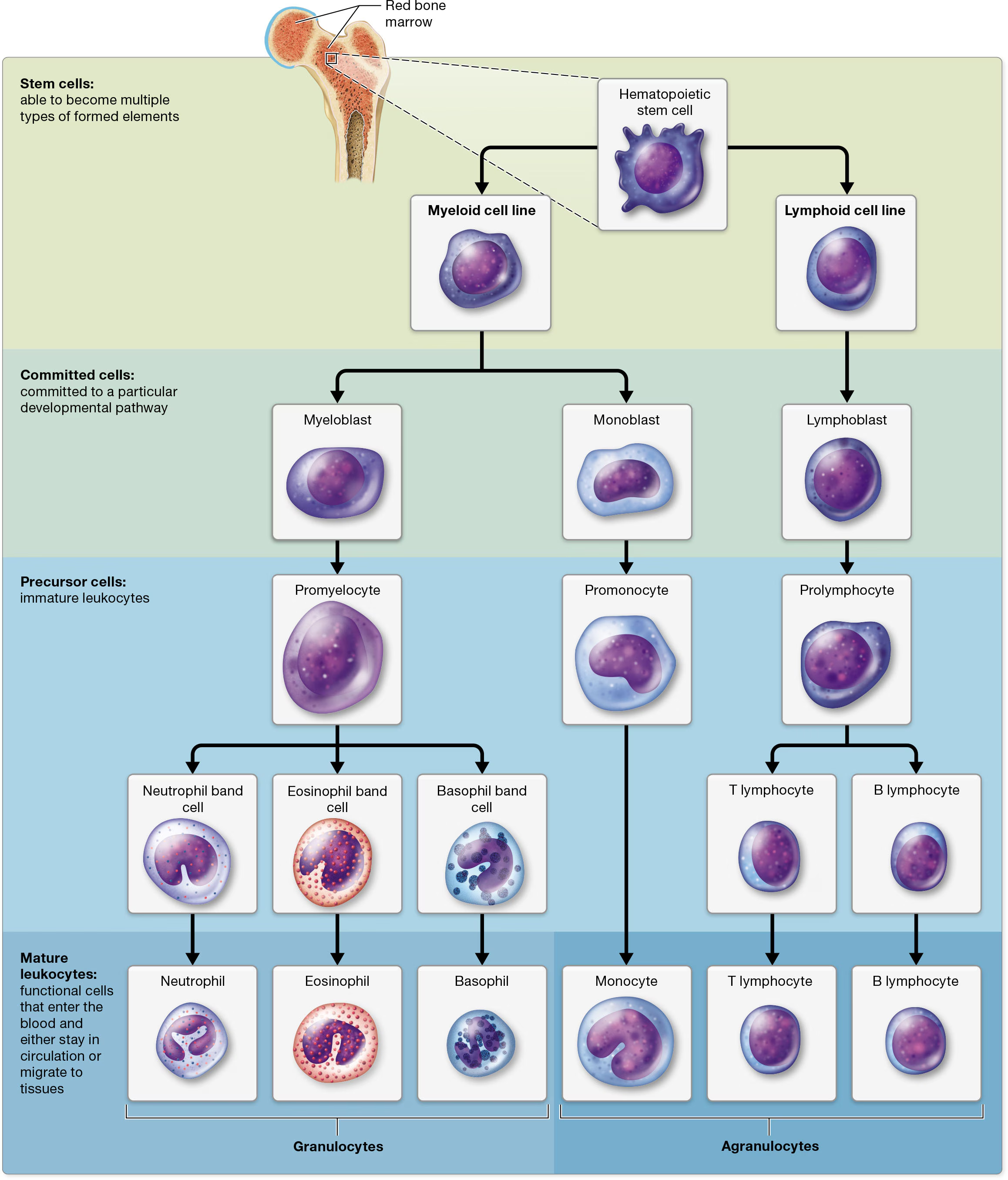
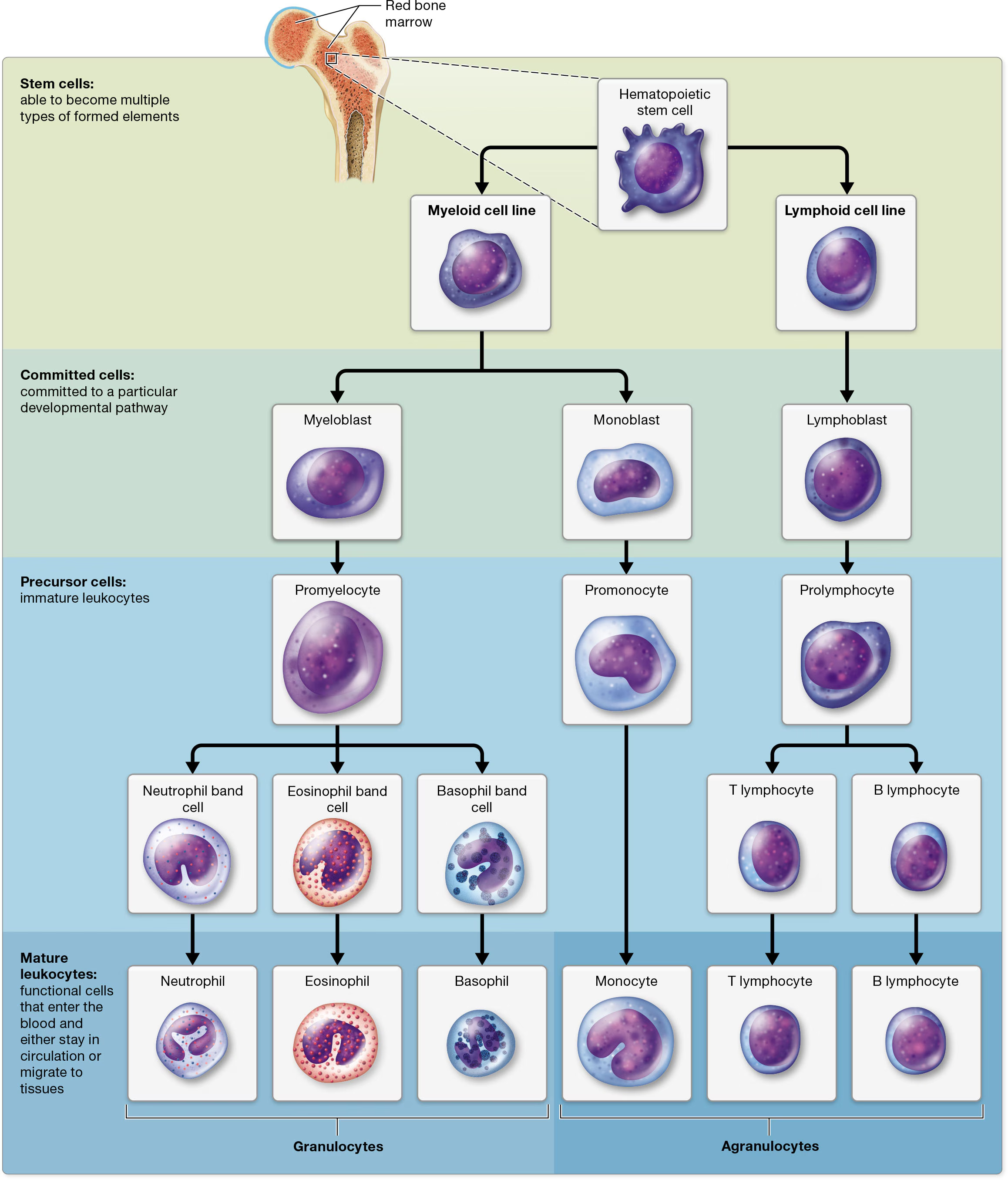
Leukocyte Formation: Leukopoiesis
all leukocytes arise from hematopoietic stem cells (HSCs)
from HSCs, they divide into 2 cell lines: Myeloid cell line (produces most of the formed elements, including erythrocytes and platelets) and Lymphoid cell line (forms lymphocytes)
Myeloid cell line:
differentiates early into blast cells that are committed to becoming monocytes or granulocytes.
Monocytes develope from
monoblasts (commited cells) that turn into
promonocytes, then finally
monocytes
Granulocytes develope from
myeoblasts that turn into
promyelocytes, then
band/stab cells (which develop into mature granulocytes that enter the bloodstream.).
If someone has an active infection, it is common to find band cells in the blood as the immune system attempts to rapidly increase the numbers of circulating leukocytes.
Lymphoid cell line:
lymphoid cell line
lymphoblast
prolymphocytes
Mature B and T lymphocytes
B lymphocytes remain in the bone marrow, whereas T lymphocytes migrate to the thymus gland in the mediastinum to complete their maturation
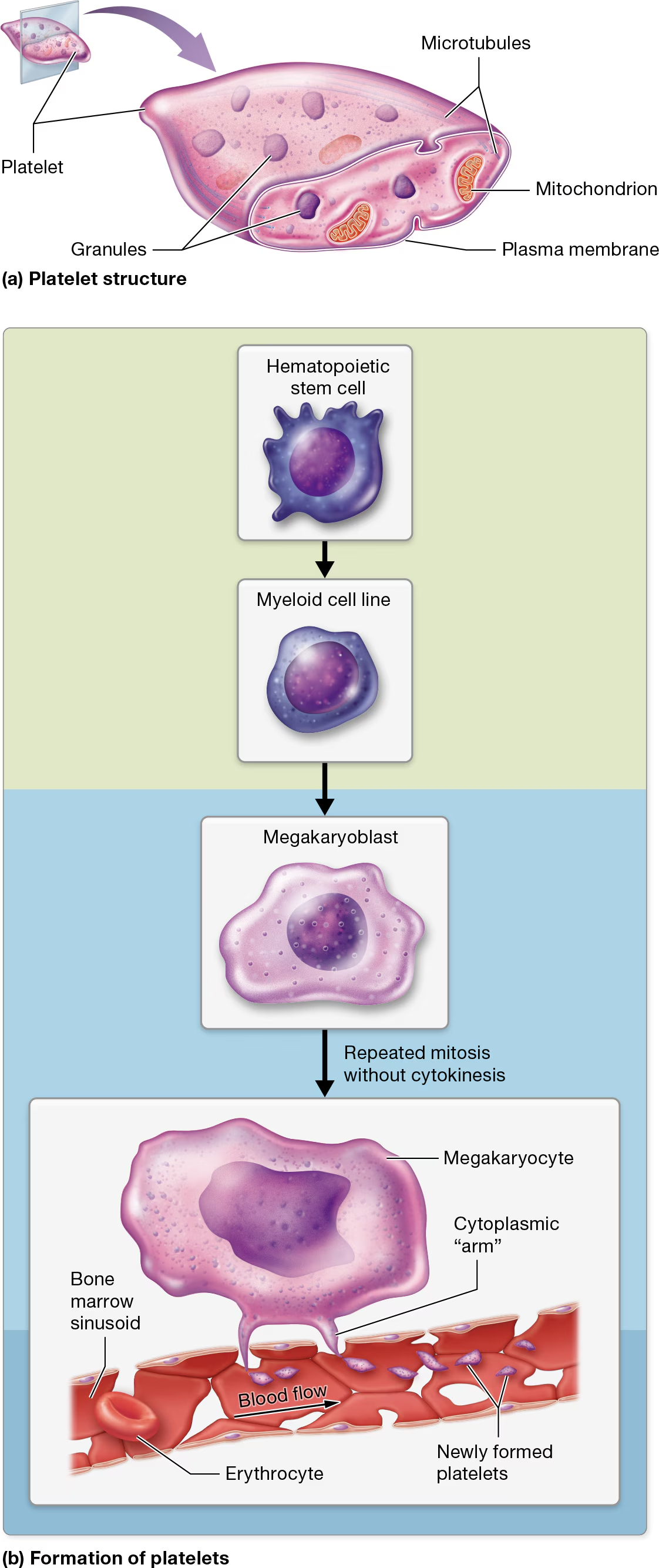
Platelets
smallest of the formed elements
involved in hemostasis - a process that stops blood loss from an injured blood vessel
Platelet characteristics
not true cells, but are fragments of cells surrounded by a plasma membrane
lack nuclei and most other organelles.
However, they do contain multiple types of granules that house clotting factors and enzymes, small numbers of mitochondria, and glycogen deposits that enable them to carry out oxidative catabolism.
These tiny structures also contain cytoskeletal components, including a system of microtubules that are associated with actin and myosin filaments.
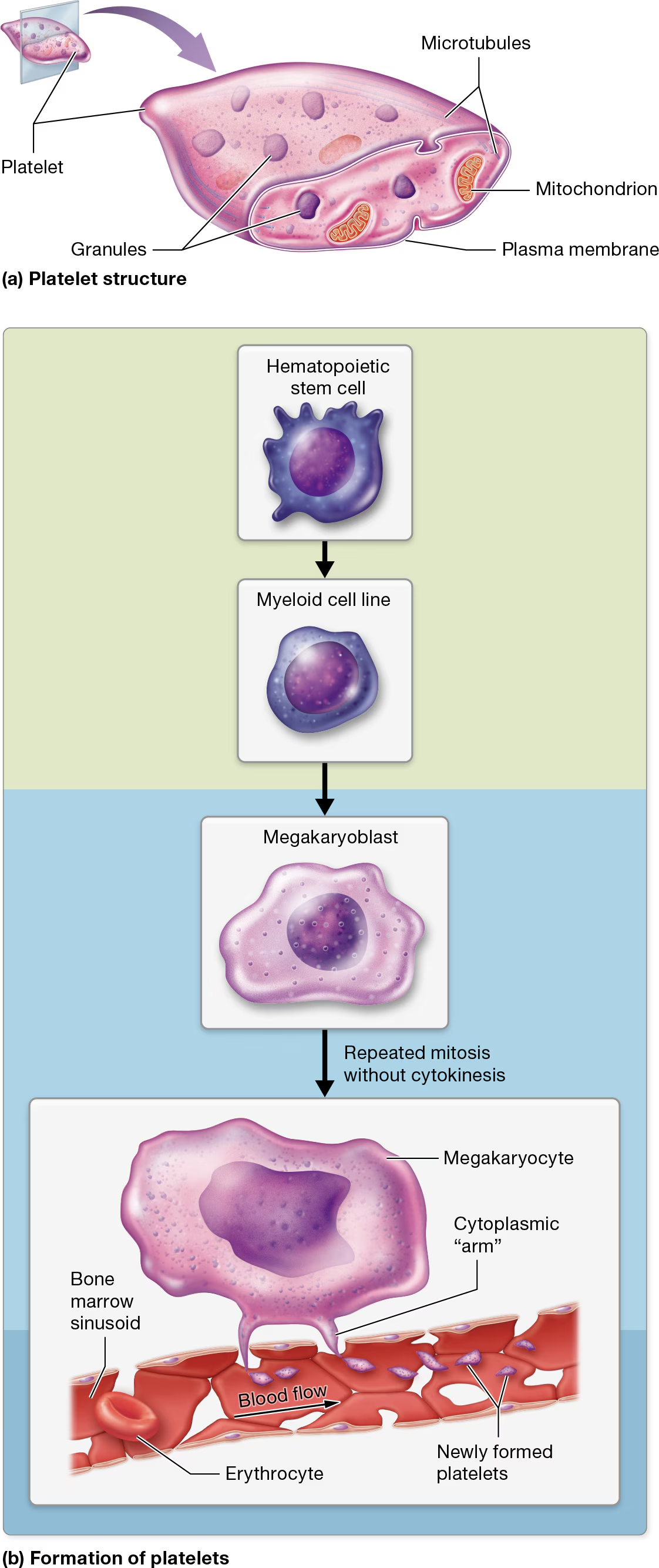
Platelet Formation
Like all formed elements, platelets begin as hematopoietic stem cells (HSCs)
myeloid cell line
differentiate into megakaryoblasts
megakaryoblasts become megakaryocytes, which undergo repeated rounds of mitosis.
However, unlike other cells, megakaryocytes do not undergo cytokinesis during anaphase and telophase of mitosis. The end result is a massive cell with multiple copies of DNA located in a single nucleus
Under the influence of hormones such as thrombopoietin, mature megakaryocytes extend ribbon-like “arms,” or extensions, filled with cytosol, granules, and several organelles. these arms extend through the clefts in the bone marrow sinusoids and into the bloodstream.
The force of the blood coursing past the arms lops off small pieces that become platelets.
Each arm can give rise to thousands of platelets.
Most remain in the general circulation, where they have a lifespan of only 7–10 days. When platelets have reached old age, the liver and spleen remove them from circulation.
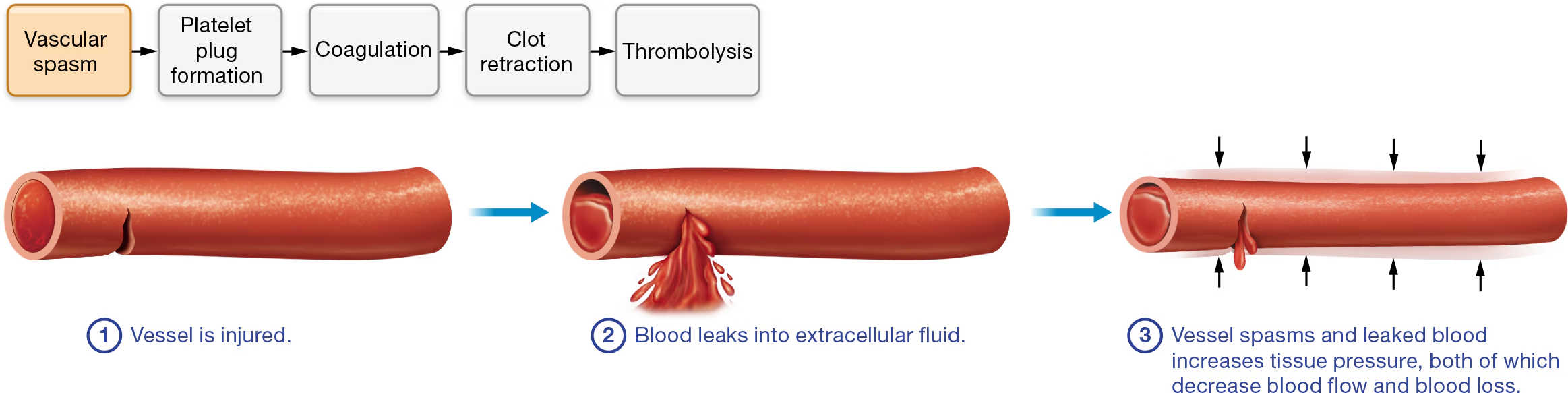
Hemostasis Part 1: Vascular Spasm
① a blood vessel is injured and ② blood leaks into extracellular fluid, two immediate responses occur: ③ spasm of the vascular smooth muscle and increased tissue pressure. Both responses decrease the blood vessel diameter.
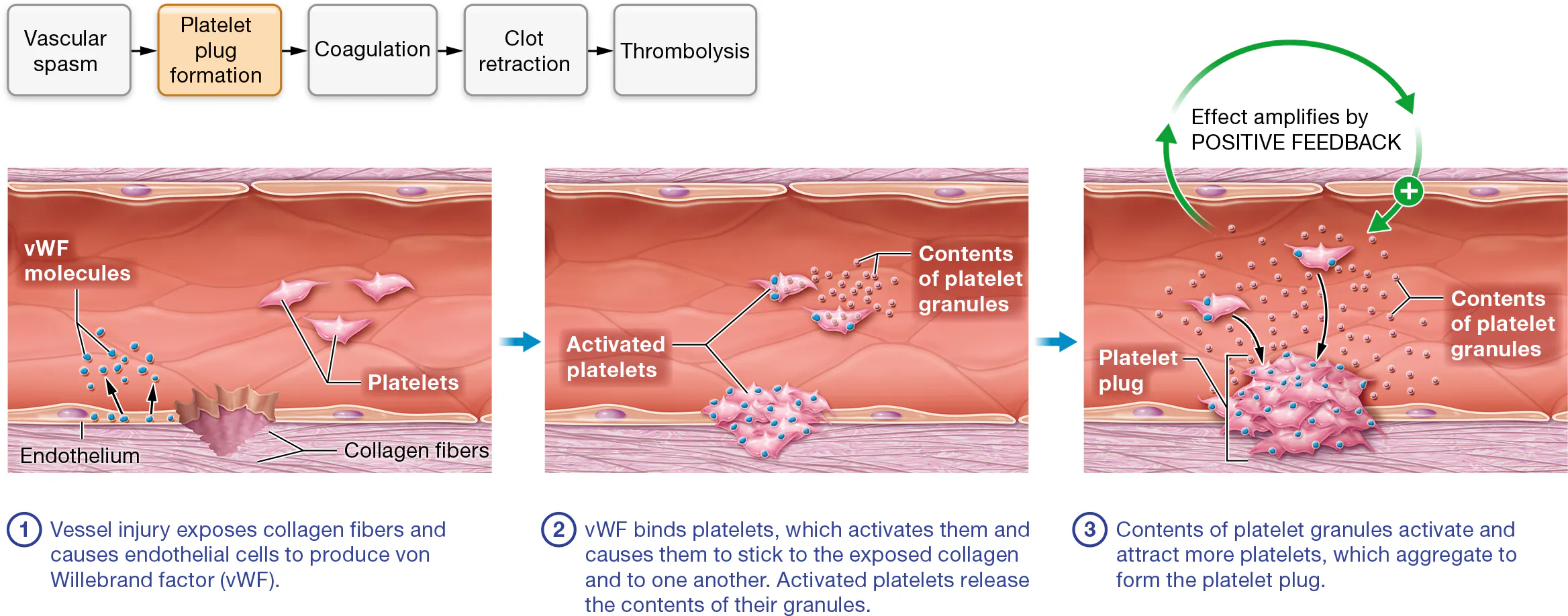
What is a platelet plug?
normally, platelets are not sticky, however platelets do adhere to an injured site.
when a vessel is injured,
collagen fibers and chemicals in the vessel’s tunica adventitia are exposed.
The injured endothelial cells release a glycoprotein called von Willebrand factor (fahn VIL-uh-brant), or vWF
vWF binds to the receptors on the surface of platelets’ plasma membranes
Together, the exposed collagen and vWF make the platelets sticky so they adhere to one another and the vessel wall
Platelet activation: Activated platelets release the contents of their granules— ATP, ADP, serotonin, calcium ions, clotting factors, and other chemicals—by exocytosis.
This effect amplifies through a positive feedback loop, as many of these factors attract and activate nearby platelets, which in turn release the contents of their granules and attract additional platelets.
The platelets drawn to the wound clump together, or aggregate, which forms the platelet plug.
This plug is short lived without something to glue it together
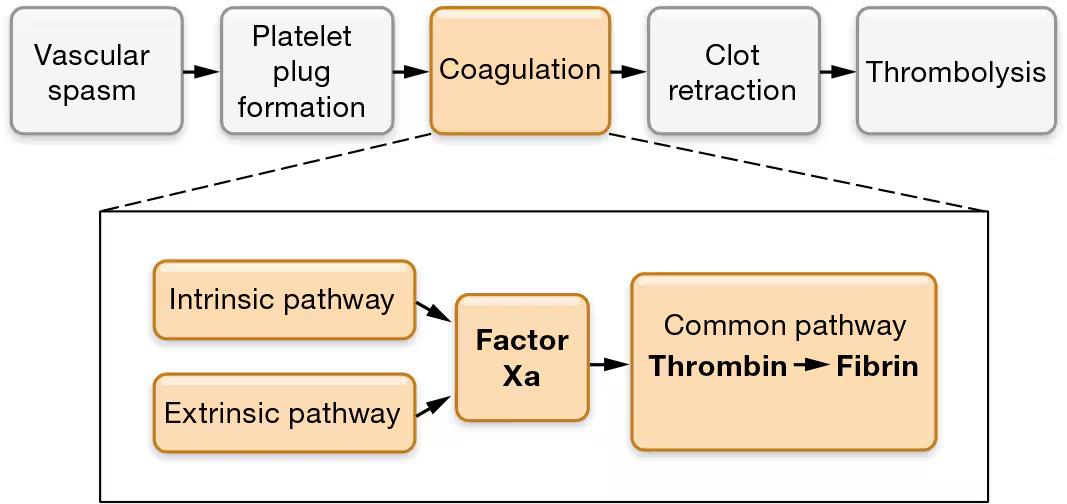
Coagulation - what is fibrin?
Fibrin (a sticky, threadlike protein) binds the platelets, endothelial cells, and other formed elements together
this converts the plug into a more solid mass thorugh coagulation
Fibrin is often found in its inactive form, where it is called fibrinogen
Fibrinogen is converted into fibrin by the coagulation cascade, a series of reactions that occur at the surface of the platelets and/or damaged endothelial cells
this “cascade” relies on clotting factors (enzymes produced by the liver)
clotting factors
the synthesis of four clotting factors—factors II, VII, IX, and X—depends on the presence of vitamin K. These vitamin K–dependent clotting factors are important targets of drug therapy that blocks coagulation.
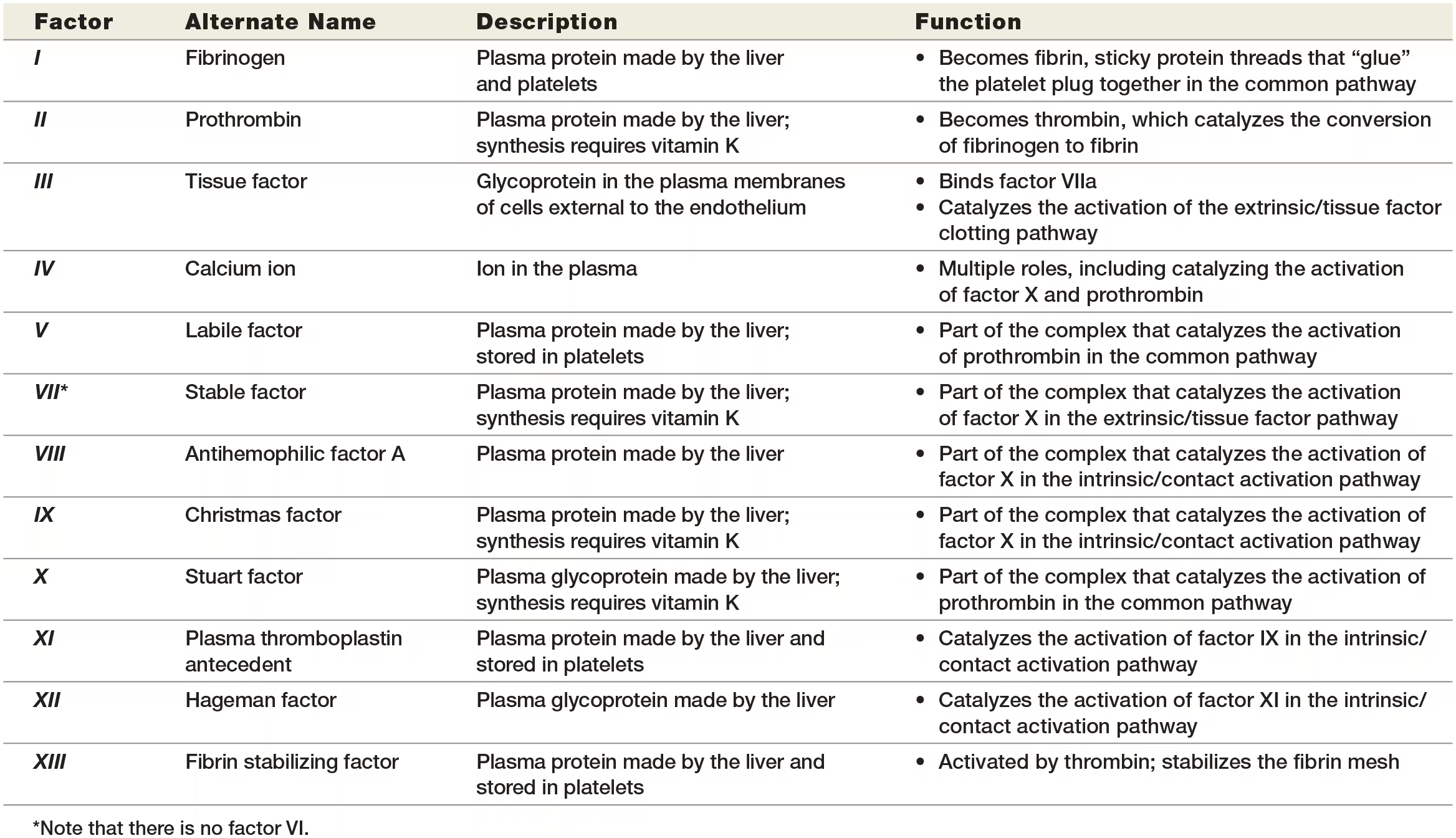
what are the 2 pathways of the coagulation cascade
Intrinsic/contact activation
extrinsic /tissue factor pathway.
Intrinsic/contact pathway
Exposed collagen fibers activate factor XII. The negative charges on the surface of the exposed collagen proteins attract and bind a clotting protein in the plasma called factor XII. Activated factor XII is known as factor XIIa (“a” stands for “activated”).
STEP 2:Factors XI and IX become activated. Factor XIIa is an enzyme that activates another clotting protein in the plasma, factor XI. Factor XIa, in turn, enzymatically activates another clotting protein, factor IX.
STEP 3:Factors IXa and VIIIa and calcium ions form an enzyme complex that activates factor X. The intrinsic/contact activation pathway ends by forming a large enzyme complex that consists of factor IXa, another protein called factor VIIIa, and calcium ions. This enzyme complex activates factor X, which becomes factor Xa. Note that calcium ions are required for the factors to associate with platelets in the platelet plug.
The intrinsic/contact activation pathway isn’t just activated by exposed collagen fibers—activation occurs any time blood contacts a surface with negative charges, including glass test tubes.
A deficiency in any clotting protein can disrupt the entire coagulation cascade.
Extrinsic/Tissue Factor Pathway
occurs simultaneously with the intrinsic/contact activation pathway, has this name because it is initiated by a factor outside (“extrinsic to”) the blood. This factor is a protein known as tissue factor
STEP 1:Subendothelial cells display tissue factor. Beneath the endothelium is a layer of loose connective tissue called the subendothelium. When an injury to the blood vessel penetrates the endothelium, it damages the subendothelium beneath it. Damaged subendothelial cells, mostly fibroblasts, display tissue factor in their plasma membranes.
STEP 2:Tissue factor activates factor VII. The clotting protein factor VII circulates in the blood in its inactive form. When it comes into contact with tissue factor, it changes in structure and becomes the active form, factor VIIa.
STEP 3:Factor VIIa, tissue factor, and calcium ions form an enzyme complex that activates factor X. Factor VIIa forms a complex with tissue factor and calcium ions that enzymatically cleaves factor X into factor Xa. The calcium ions play the same role here as they did in the intrinsic/ contact activation pathway: They are required for the interaction of factors with platelets in the platelet plug.
A deficiency in any clotting protein can disrupt the entire coagulation cascade.
common pathway
The end result of both pathways is the same: activation of factor X to the active enzyme factor Xa.
At this point, the two pathways converge to the common pathway.
STEP 4:Factors Xa and Va and calcium ions form prothrombin activator, which converts prothrombin into thrombin. The common pathway begins with the formation of prothrombin activator, a large enzyme complex that consists of factor Xa, another protein called factor Va, and calcium ions. Prothrombin activator converts the inactive protein prothrombin to the active enzyme thrombin.
STEP 5:Thrombin turns fibrinogen into fibrin, which “glues” the platelet plug together. In the final step of the coagulation cascade, thrombin acts on the inactive protein fibrinogen, which is both present in plasma and released by platelets. Thrombin converts fibrinogen into fibrin, its active form. The fibrin threads form a mesh that glues together the platelet plug, other formed elements, and damaged endothelial cells, sealing the damaged blood vessel.
why does thrombin catalize the activation of factors V and VIII?
the enzymes have a small amount of activity before thrombin is produced. As this activity leads to thrombin production, thrombin acts on the factors to convert them to their active forms, which accelerates thrombin production.
This is a POSITIVE FEEDBACK LOOP
how is thrombin’s positive feedback loop self-limiting?
clotting factors are rapidly inactivated by enzymes in plasma, so their action is fairly brief and limited to the site of injury.
Second, fibrin inhibits the production of thrombin via a negative feedback loop, so thrombin activity decreases as the fibrin level increases.
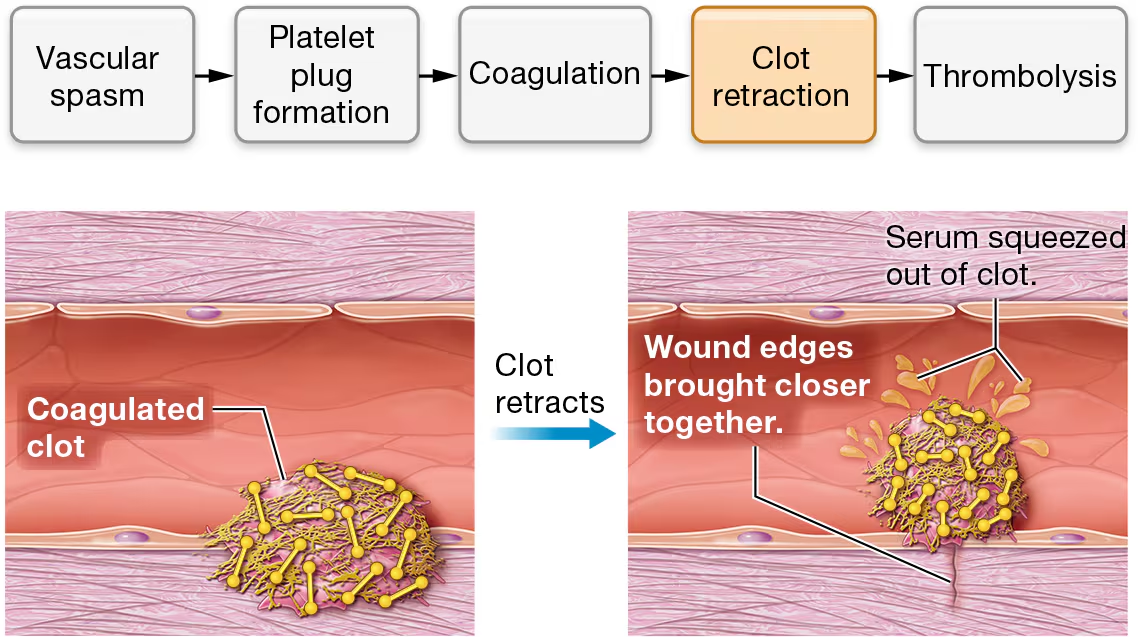
clot retraction
the actin and myosin fibers in the platelets contract. this is known as clot retraction.
this brings the endges of the wounded vessel closer together, much like stitches in skin would
Clot retraction also squeezes serum (SEER-um)—a fluid consisting of plasma minus the clotting proteins—out of the clot. (Think of it as squeezing water out of a wet rag.)
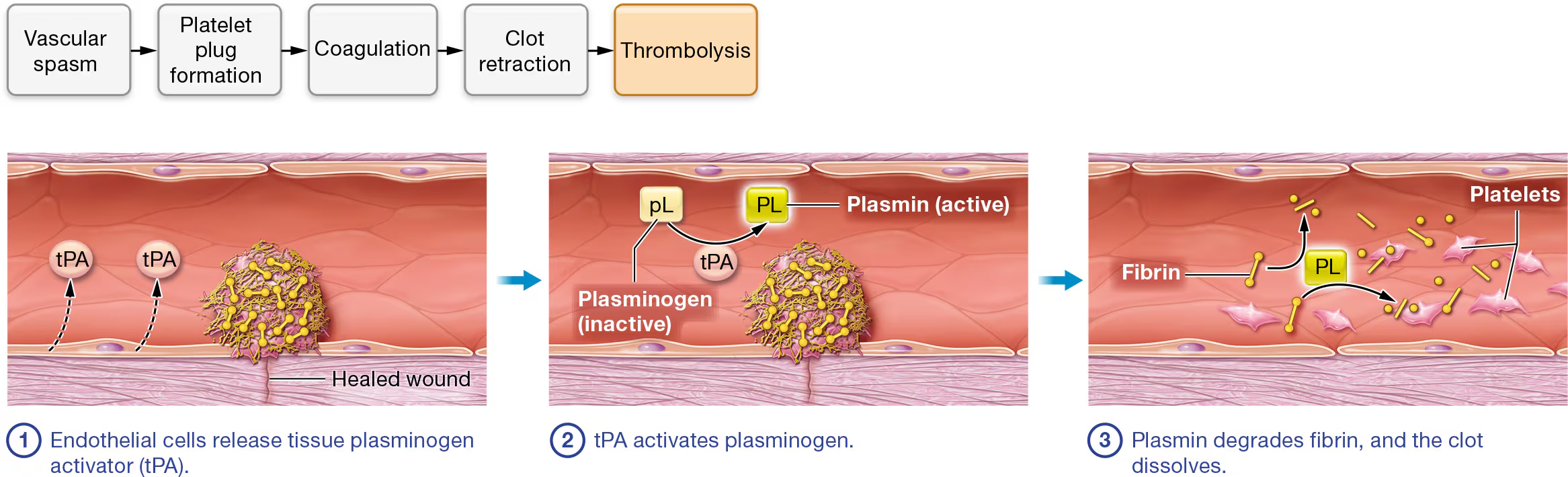
thrombolysis
once the wound is healed, the clot is no longer needed, so thrombolysis occurs.
begins with fibrinolysis
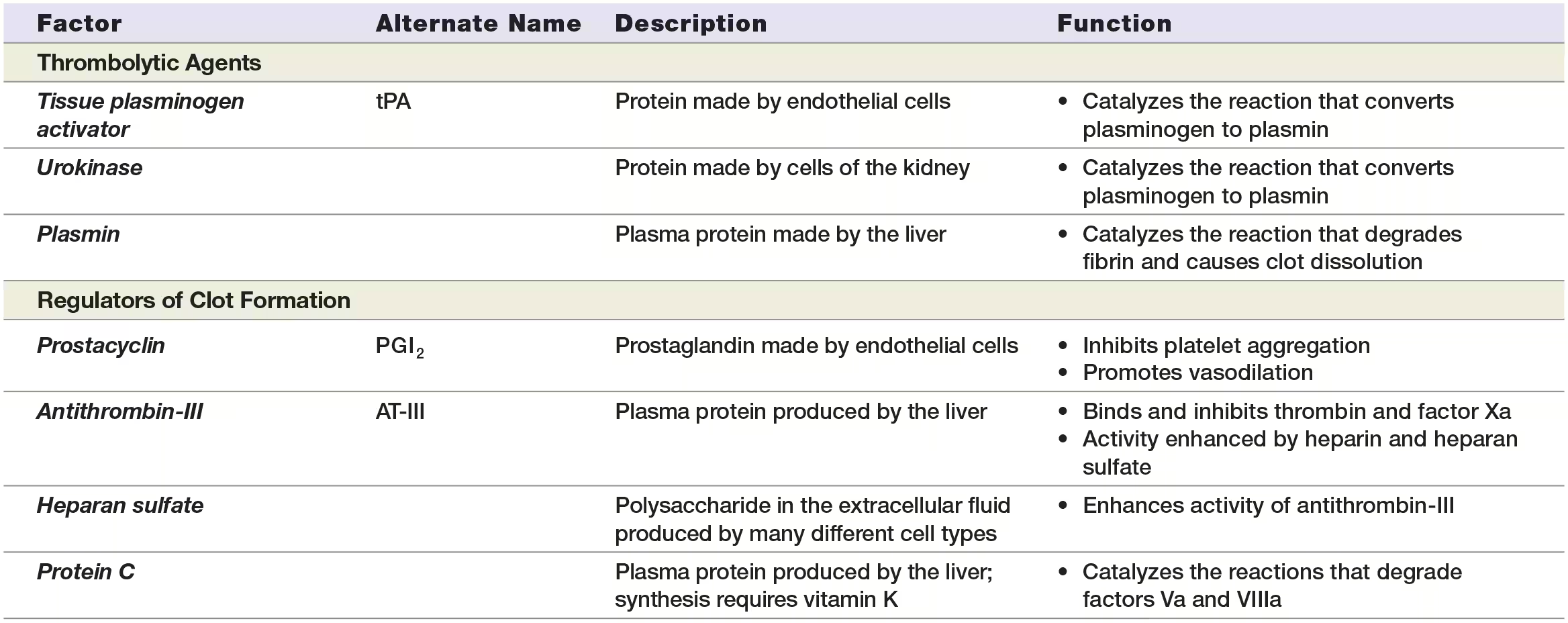
STEP 1:Endothelial cells release tissue plasminogen activator (tPA). Thrombolysis begins when healed endothelial cells produce and release the enzyme tissue plasminogen (plaz-MIN-oh-jen) activator, or tPA. Thrombolysis can also be initiated by a similar enzyme called urokinase (yoo-roh-KY-nayz), which is produced by cells of the kidney and found in the plasma and interstitial fluid.
STEP 2:tPA activates plasminogen. The inactive enzyme plasminogen, which is normally found in plasma, has a high affinity for fibrin proteins and binds them as they are incorporated into the blood clot. As a result, every blood clot contains a significant amount of plasminogen. When tPA contacts plasminogen, it catalyzes the reaction that converts it to the active enzyme plasmin.
STEP 3:Plasmin degrades fibrin, and the clot dissolves. Plasmin catalyzes the reaction that degrades both fibrin and fibrinogen. This causes the remaining components of the clot to dissociate from the endothelium.
Regulation of Clotting
Endothelial cells produce chemicals that regulate the first and second stages of clot formation. Two of these chemicals are prostacyclin (prah-stuh-SY-klin), which inhibits platelet aggregation, and nitric oxide, which causes vasodilation.
Prostacyclin is a prostaglandin, a group of chemicals with many functions, including triggering inflammation. Anti-inflammatory medications such as ibuprofen inhibit prostaglandin formation. Some of them also inhibit prostacyclin formation, a fact that accounts for the increased risk of blood clots with these drugs
what are examples of anticoagulations produced by endothelial cells and hepatocytes?
Antithrombin-III. One of the most important anticoagulants, the protein antithrombin-III (AT-III), binds and inhibits the activity of both factor Xa and thrombin. It inhibits thrombin that has already formed and prevents the formation of new thrombin.
Antithrombin-III. One of the most important anticoagulants, the protein antithrombin-III (AT-III), binds and inhibits the activity of both factor Xa and thrombin. It inhibits thrombin that has already formed and prevents the formation of new thrombin.
Protein C. Active protein C catalyzes the reactions that degrade factors Va and VIIIa. To become active, protein C requires another protein, called protein S.
disorders of clotting
There are two types of clotting disorders: (1) bleeding disorders, when the blood is unable to clot, and (2) hypercoagulable conditions, when clots form at improper times and tissue locations.
bleeding disorders
often result from clotting protein deficiencies; for example, hemophilia A is caused by a shortage of factor VIII, and hemophilia B by inadequate factor IX. Many bleeding disorders can be treated by replacing the missing clotting factor or protein with periodic infusions.
hypercoagulable condition
can result in the formation of an inappropriate clot, a condition called thrombosis. The clot, or thrombus (plural, thrombi), is dangerous because it can obstruct blood flow through a vessel. In addition, a piece of the thrombus, called a thromboembolus (plural, thromboemboli, often shortened to emboli), may break off and lodge in smaller vessels
Thrombi form most often in deep veins of the legs, leading to deep vein thrombosis, or DVT. The most dangerous complication of DVT is pulmonary embolism, in which emboli break off and lodge in the vessels of the lungs. A common risk factor for DVT is prolonged immobility,
Other conditions that increase the tendency to clot include surgery, trauma, abnormal clotting factors, anticoagulant deficiencies, and pregnancy. In addition to DVT, thrombi and emboli can block the vessels of the brain, resulting in a stroke, or the heart, causing a myocardial infarction
Occasionally, it’s necessary to attempt to dissolve the clot with a thrombolytic agent. The enzyme tPA or urokinase is used when blood flow must be restored rapidly to prevent tissue damage, as, for example, when thrombi or emboli have caused a stroke or heart attack.
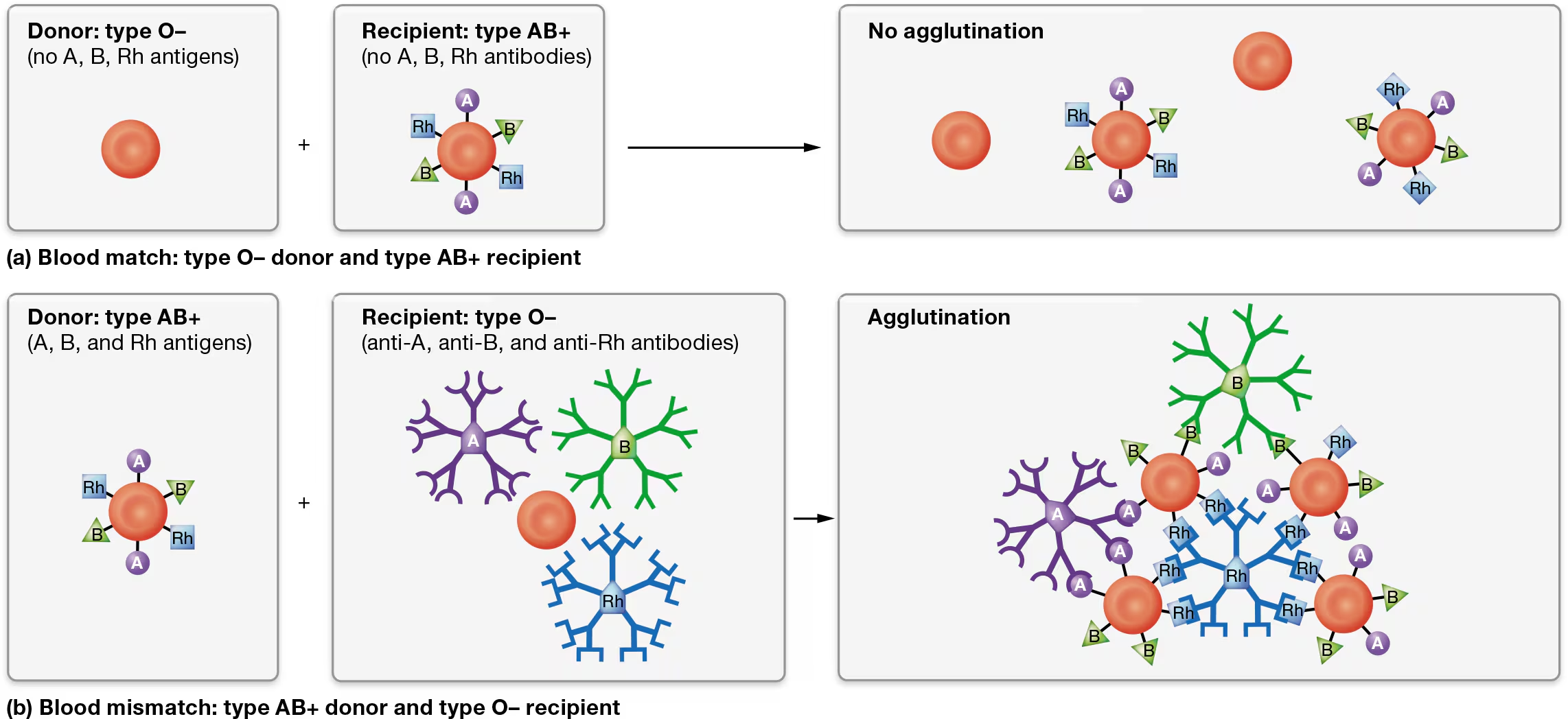
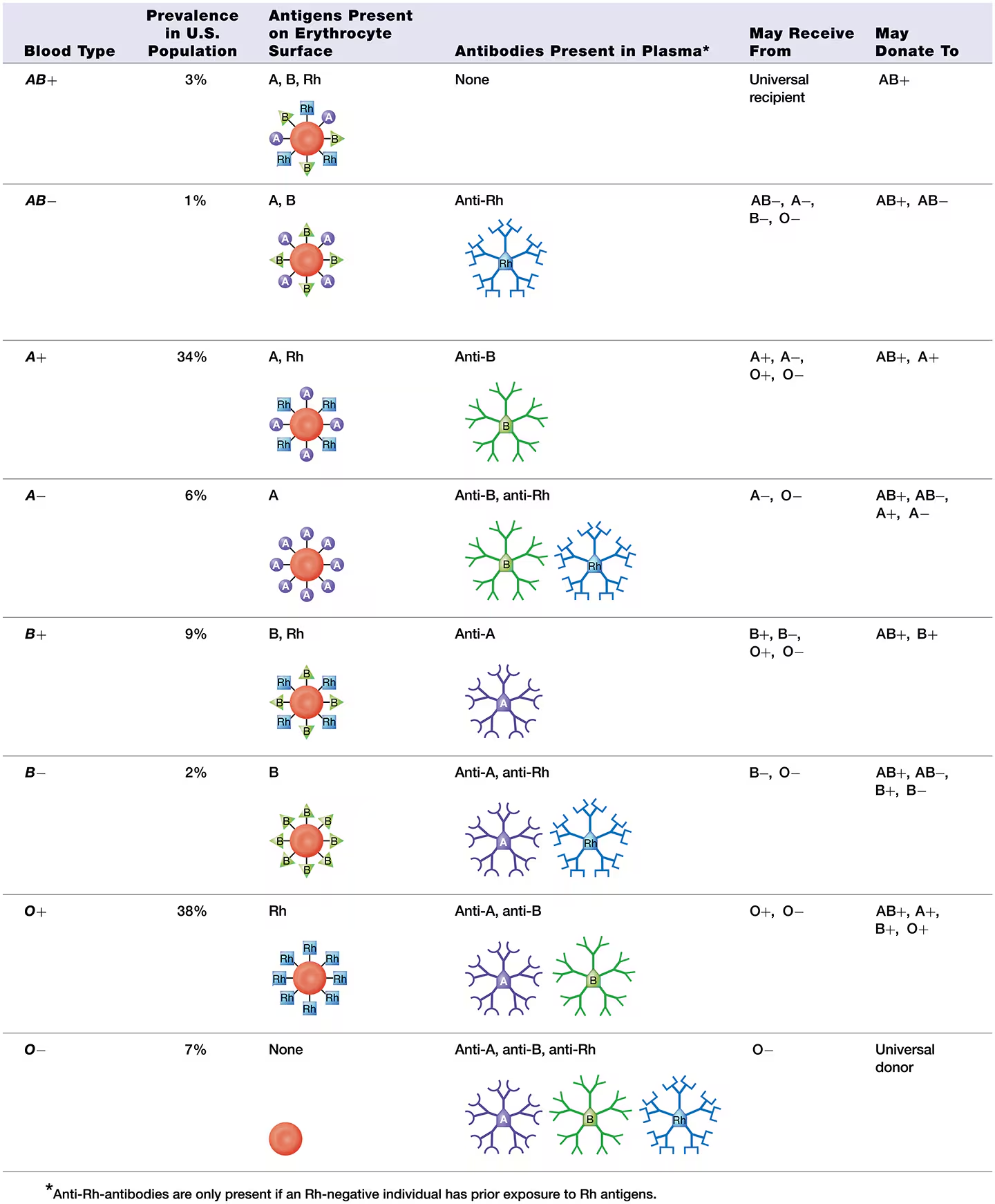
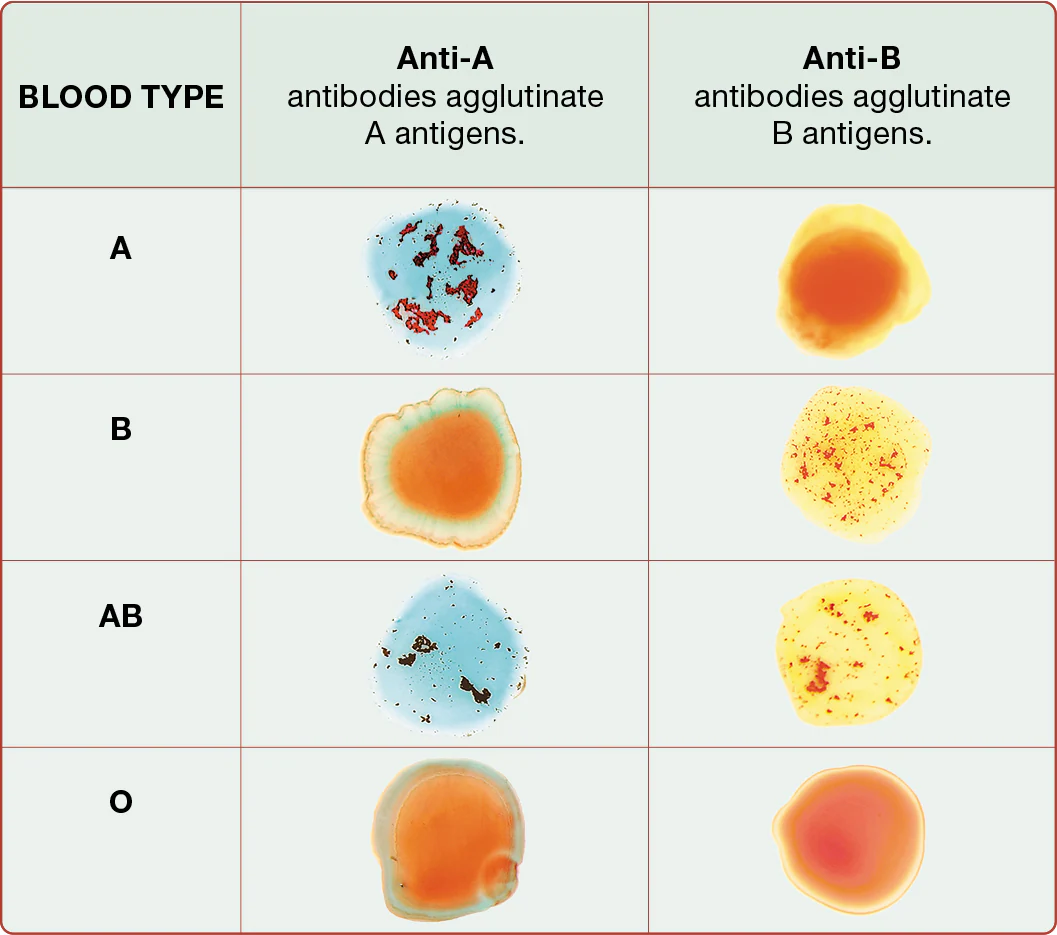
blood typing
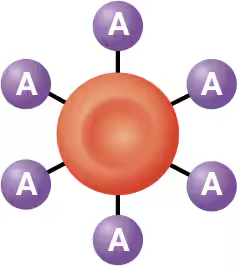
there are 2 possible antigens in the ABO blood group - A antigen and B antigen
type A blood
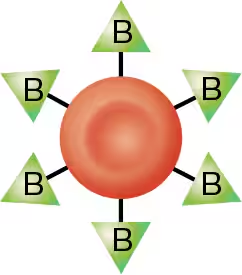
type B blood
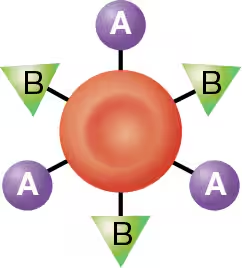
AB blood
type O blood - means A and B antigens are absent

Rh blood group
features the Rh group, also known as the D antigen
Individuals with an Rh antigen on their erythrocytes are Rh-positive (abbreviated “Rh+”)
those lacking an Rh antigen are Rh-negative (abbreviated “Rh−”).
what is the most common blood type and what is the leaset common blood type
Type O+ is the most common blood type, present in about 38% of the U.S. population. ‘
AB− is the least common, found in only about 1% of the U.S. population.
how to determine blood type
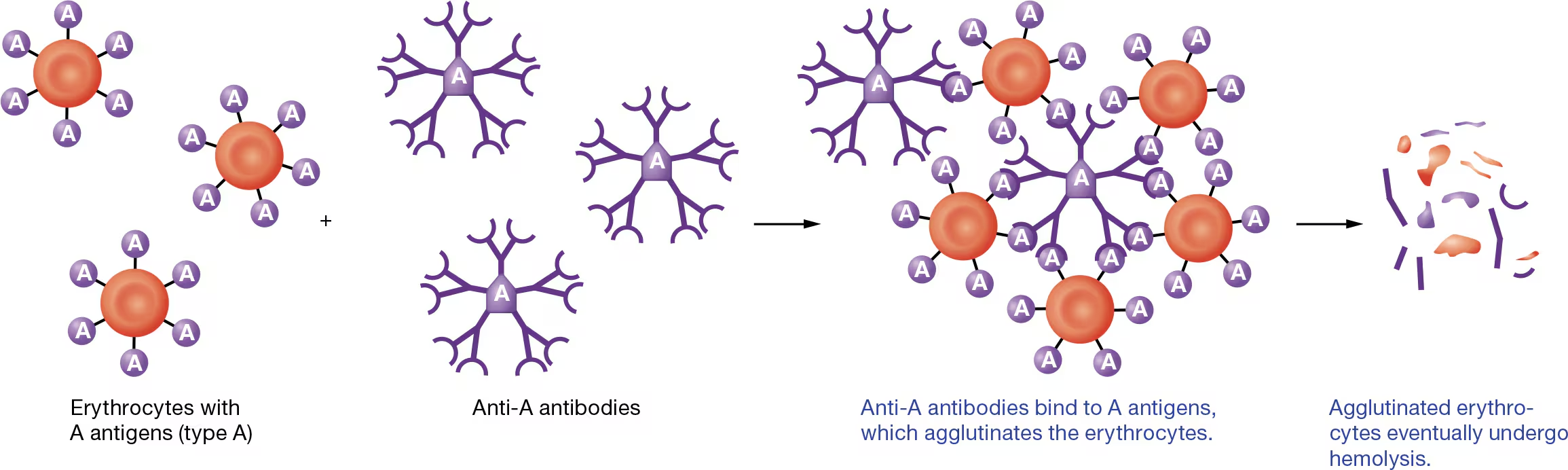
by using antibodies that recognize and bind to individual antigens on erythrocytes
As you can see in Figure 19.18, antibodies cause bound antigens to clump together, or agglutinate (uh-GLOO-tuh-nayt). For this reason, antibodies are sometimes called agglutinins. Ultimately, agglutination promotes destruction of the erythrocytes, a reaction known as hemolysis
blood transfusions
Note that anti-A and anti-B antibodies are pre-formed, meaning they are present in the plasma even if the individual has never been exposed to those antigens.
Anti-Rh antibodies, however, are produced only if a person has been exposed to blood containing Rh antigens.
For this reason, an Rh− individual generally has no anti-Rh antibodies unless he or she has been exposed (sensitized) to Rh+ erythrocytes.
This has particular relevance for pregnant Rh− women,
example
Type B- blood donor
can only donate to the blood types that lack anti-B antibodies, and so are a match for Sue’s blood, are types B+, B−, AB+, and AB−.
All the other blood types have anti-B antibodies that will agglutinate her donated erythrocytes. This agglutination, known as a transfusion reaction, destroys the donor erythrocytes, possibly leading to kidney failure and death.