Inorganic & Organic Chemistry Studying Unit 1
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Protons charge, location, and mass
+, nucleus, 1 amu
Neutrons charge, location, and mass
=, nucleus, 1 amu
Electrons charge, location, and mass
-, outside the nucleus, ~0amu
Isotope
Atoms of an element with differing #’s of neutrons
Ion
Have an unequal # of protons and electrons
Covalent bonds
A bond between two atoms that share a pair of valence electrons
Ionic bonds
A bond between two oppositely charged ions
Hydrogen bonds
A weak attraction between two polar covalent molecules
Hydrogen
1, 1, 1
Carbon
6, 4, 4
Nitrogen
7, 5, 3
Oxygen
8, 6, 2
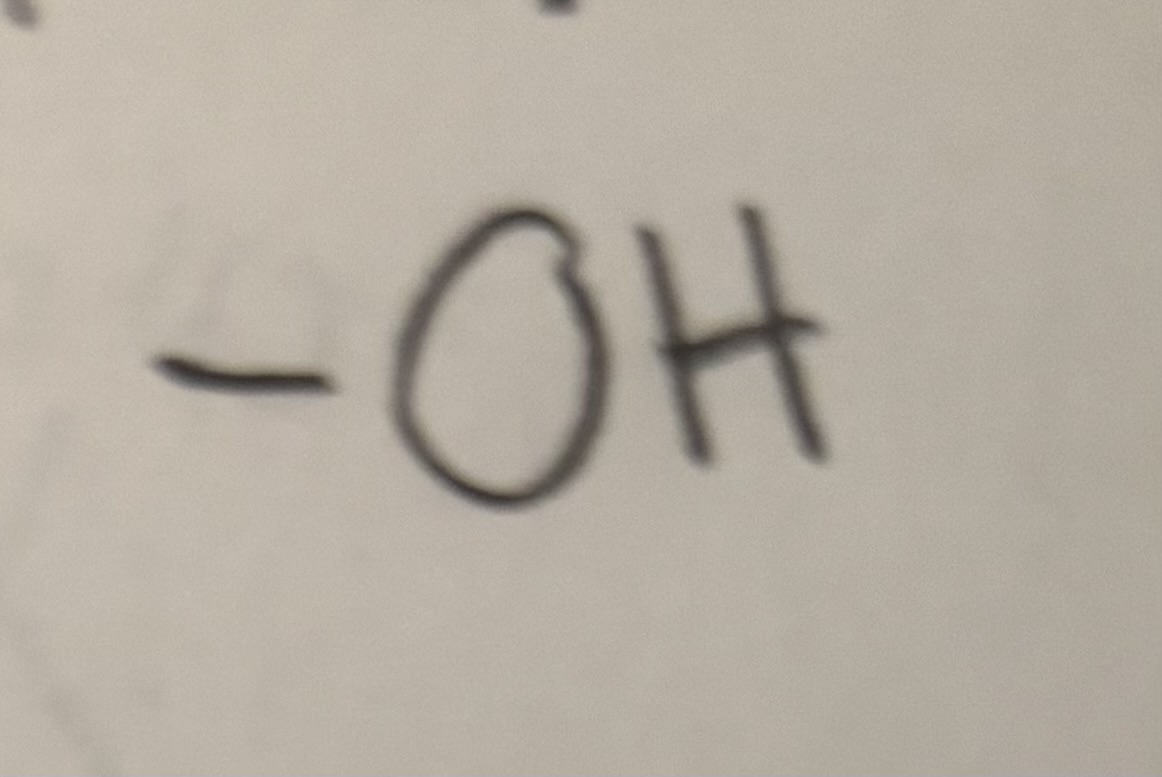
Hydroxyl
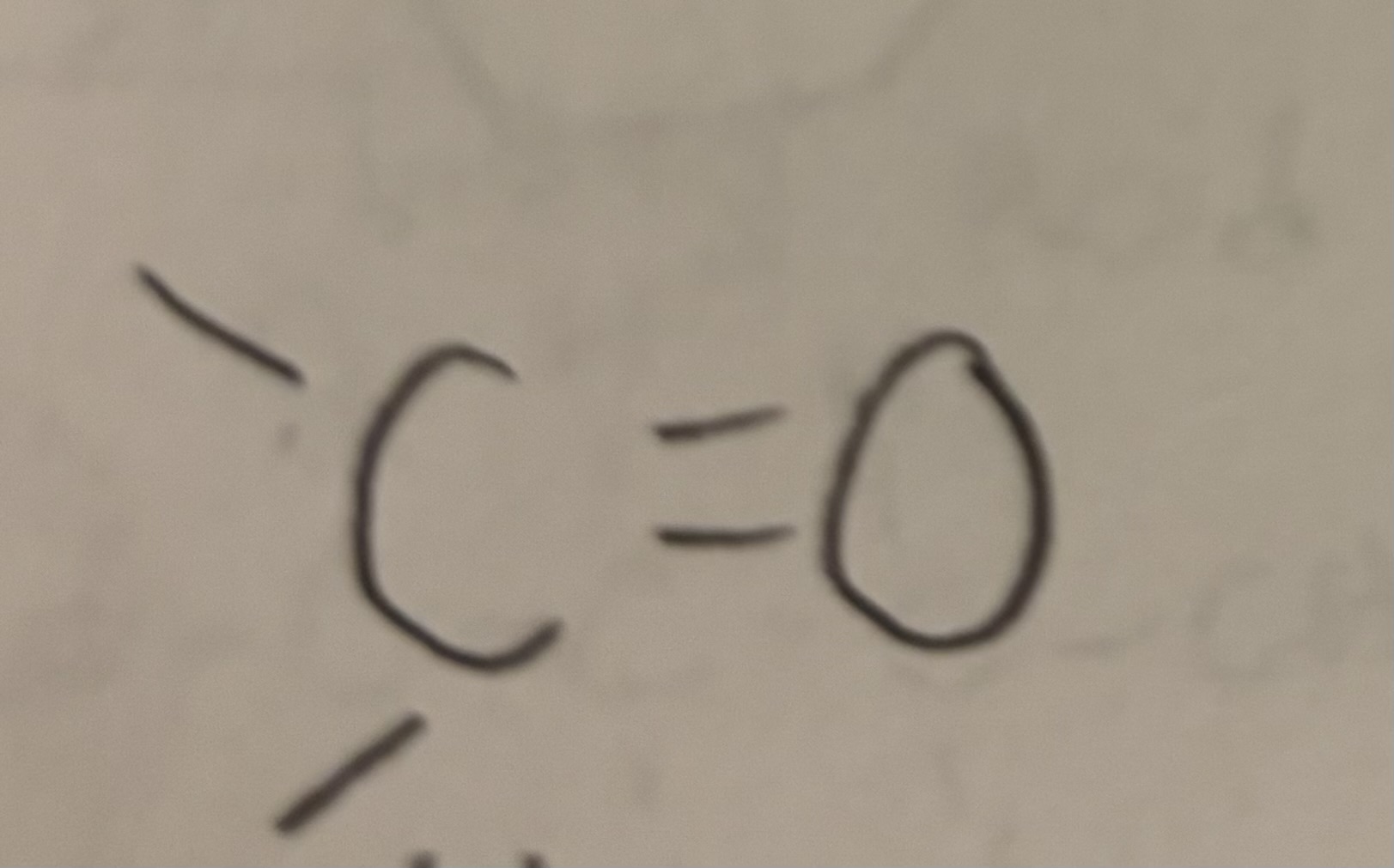
Carbonyl
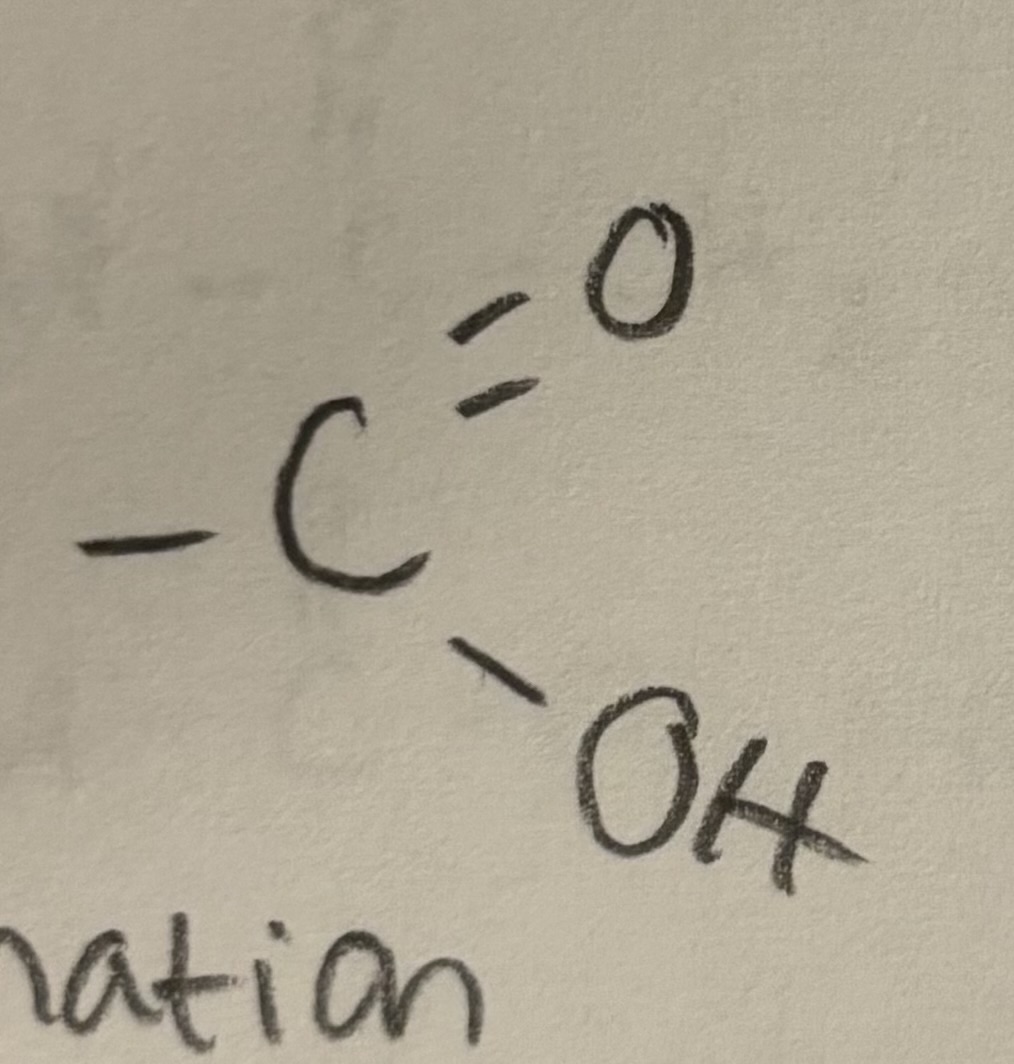
Carboxyl
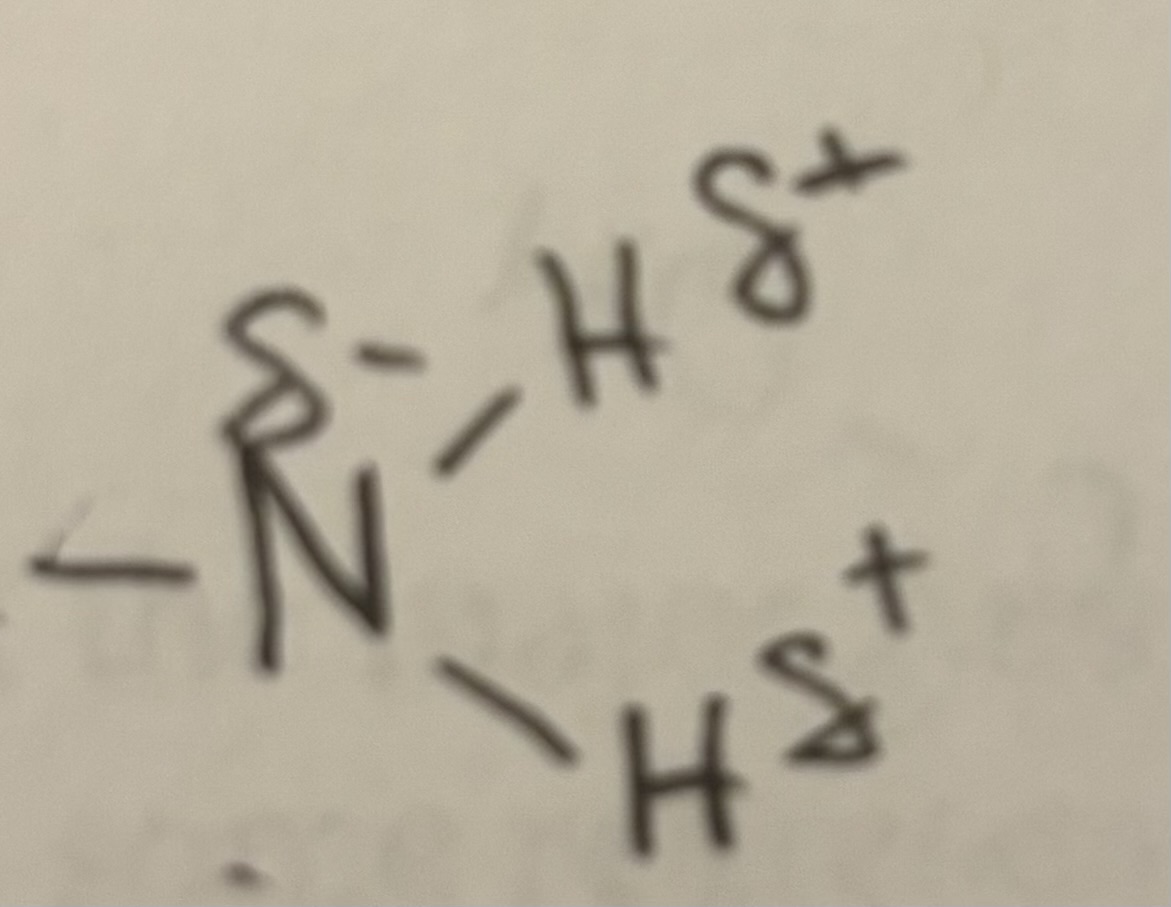
Amino
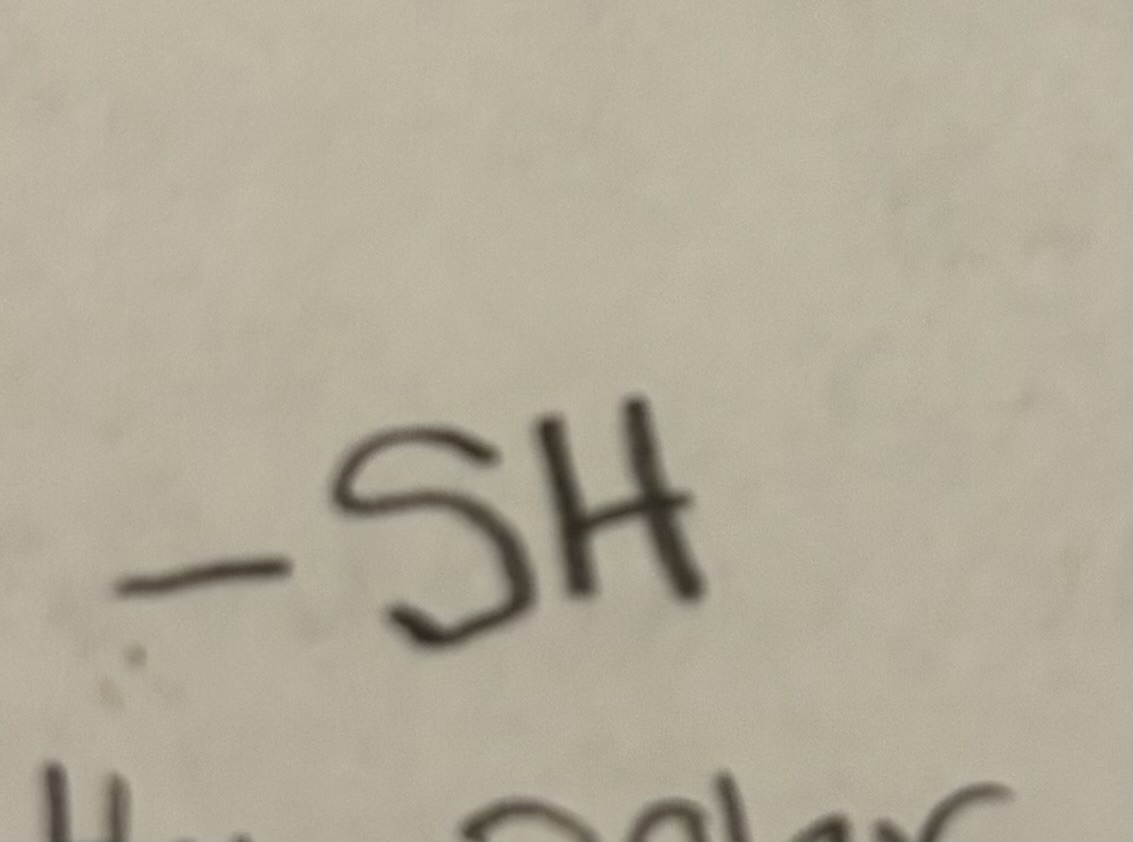
Sulfhydryl
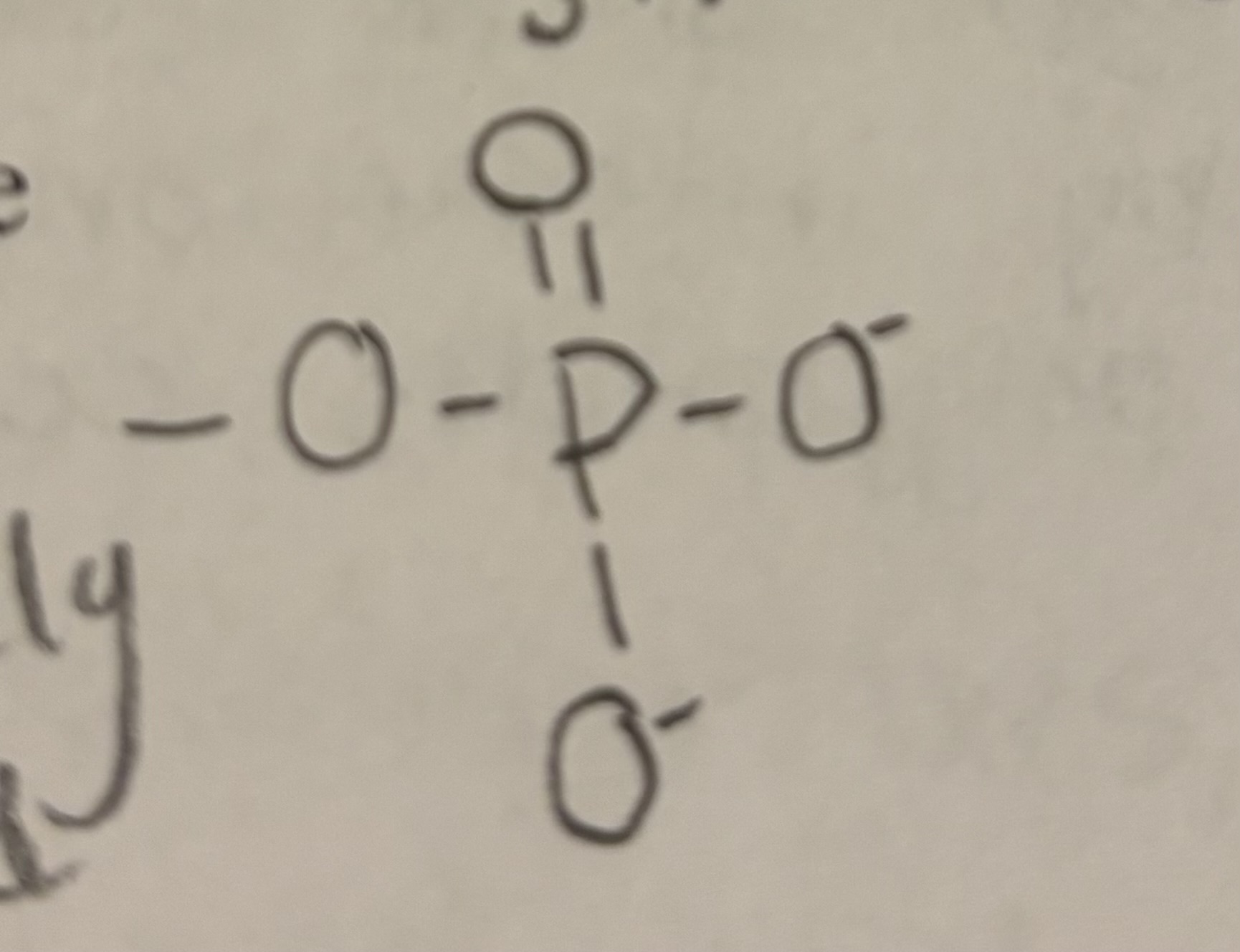
Phosphate
Primary structure
Single chain
Secondary structure
Folded sheet
Tertiary structure
Primary and secondary/the shape
Quaternary structure
Tertiary’s linked together
Carbohydrate functions
Energy and structure
Protein functions
Movement, transport, structure, communication between cells
Nucleic Acid functions
Genetic information, translate and transcription
Lipid functions
Storage, cell membrane structure, protection/insulation
How to find the # of electrons in the valence shell
Atomic # minus 2
How to find the # of unpaired electrons in the valence shell
The # of electrons subtracted from 8