Chemistry Tests
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
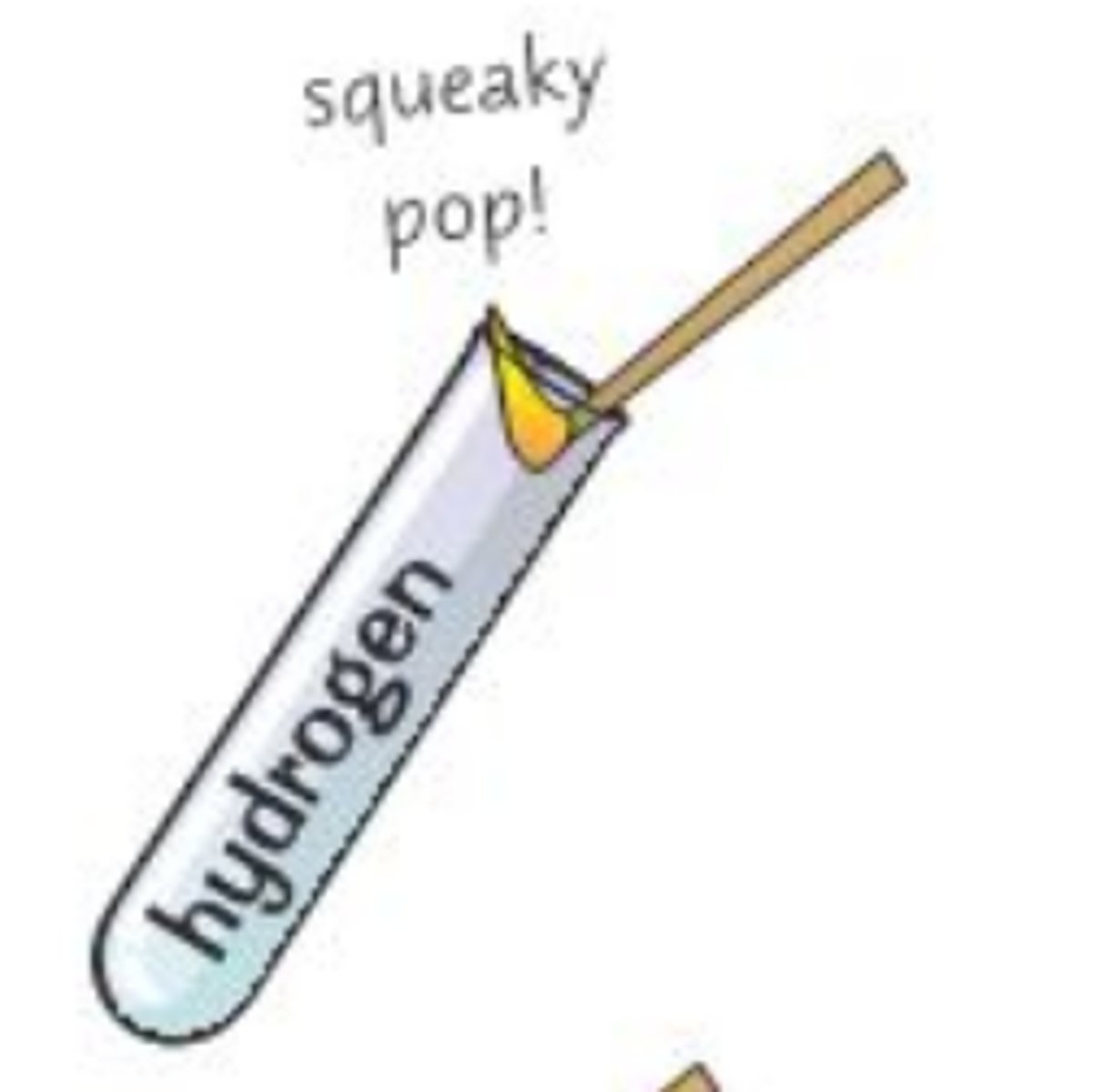
Hydrogen Test
lit splint at mouth of test tube -> squeaky pop sound
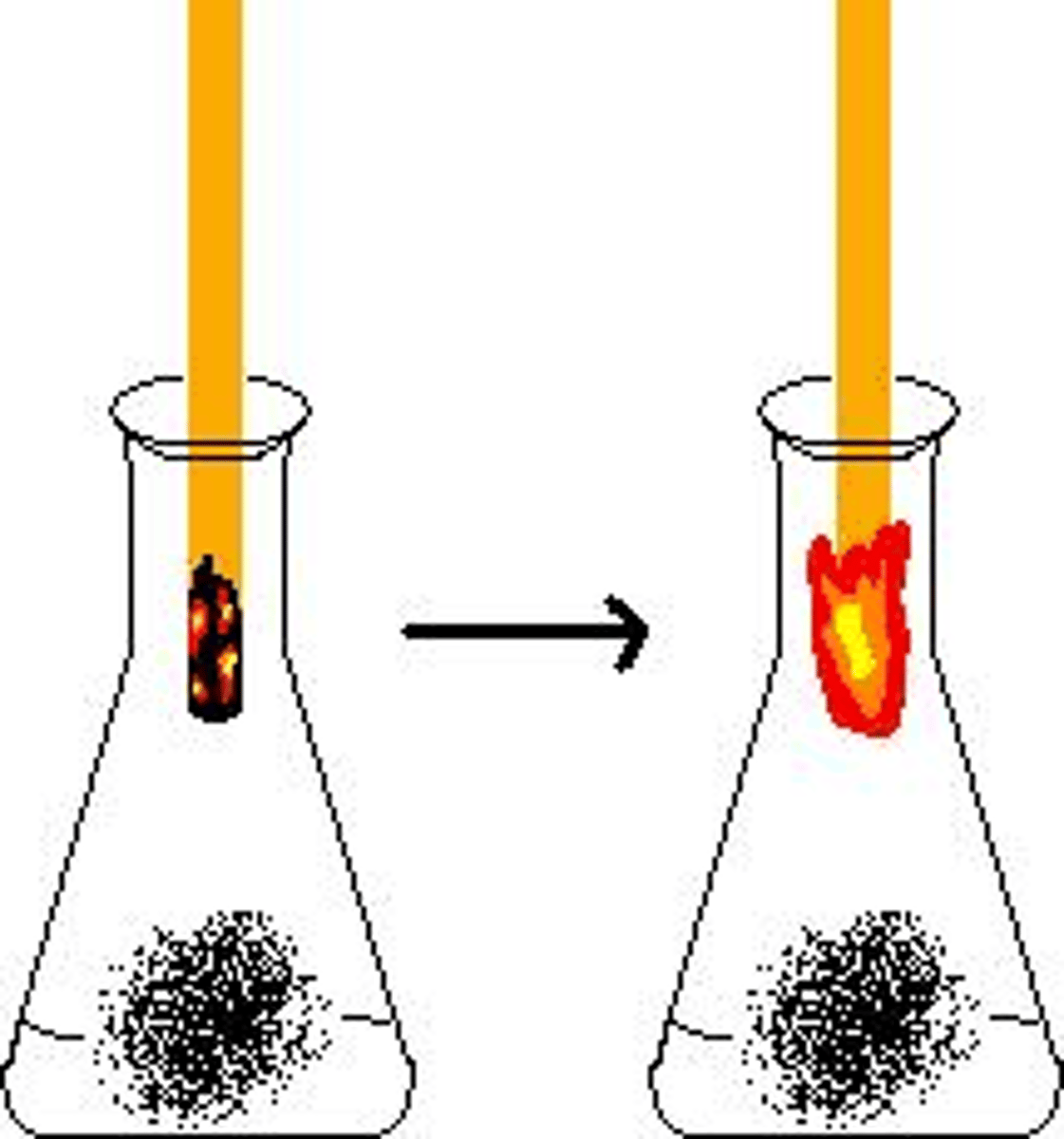
Oxygen Test
glowing splint at mouth of test tube -> relights
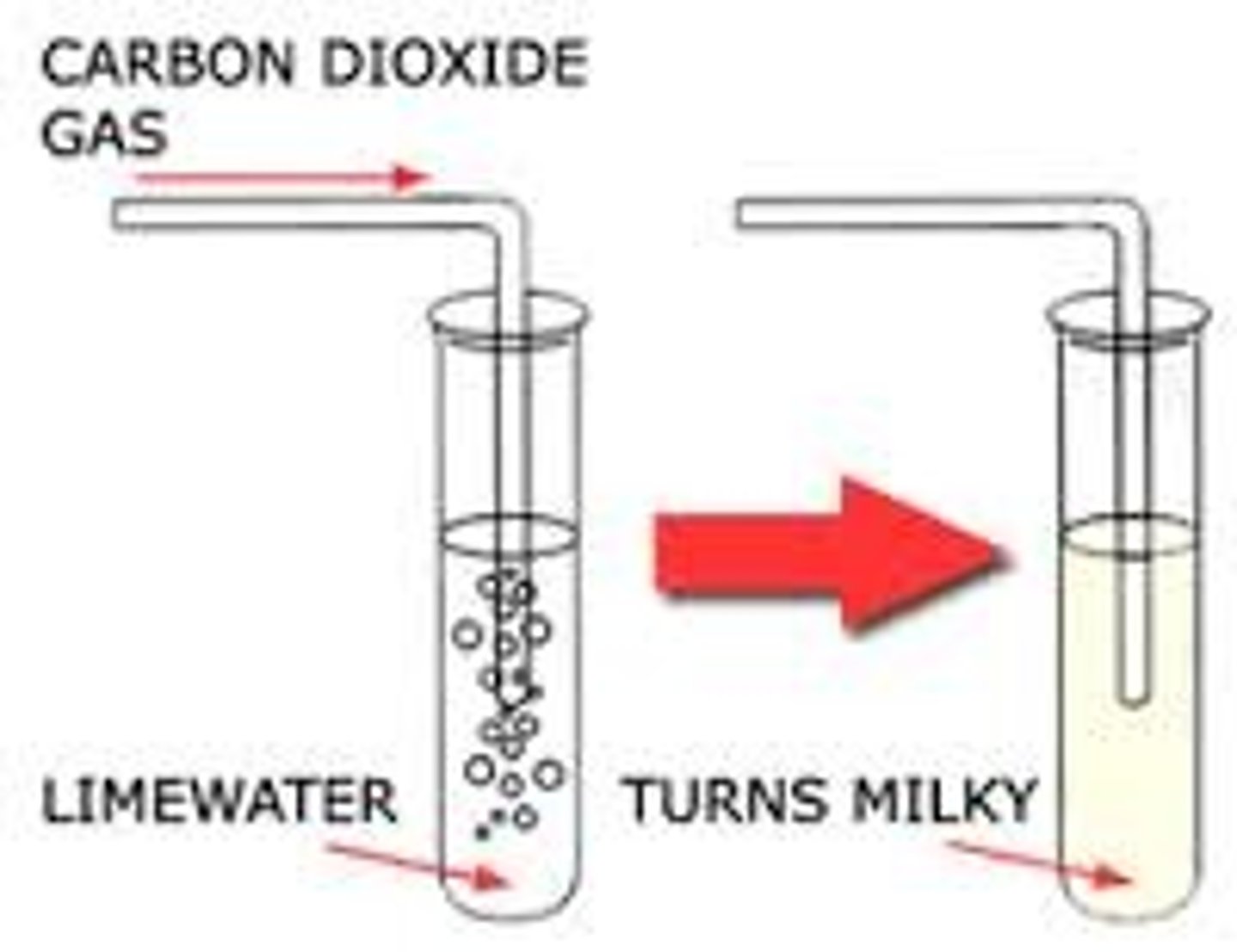
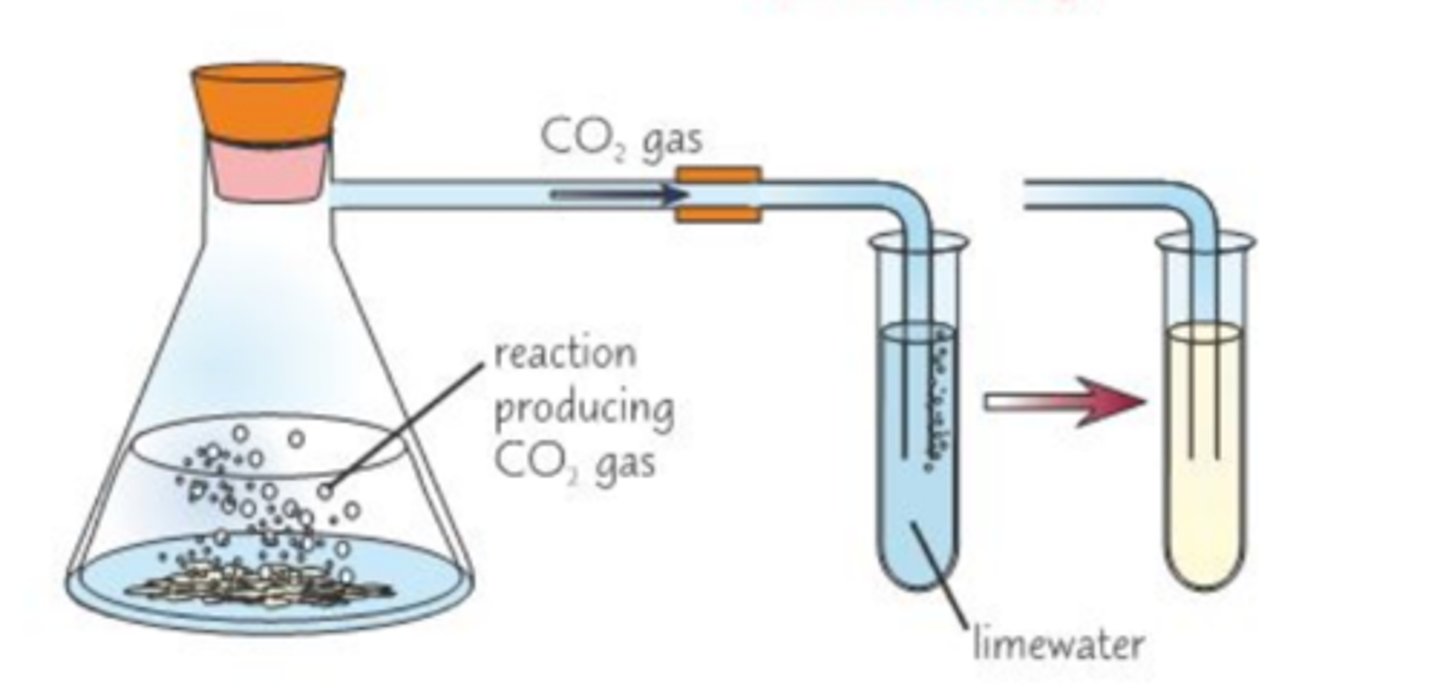
Carbon Dioxide Test
delivery tube -> limewater tube -> limewater goes cloudy
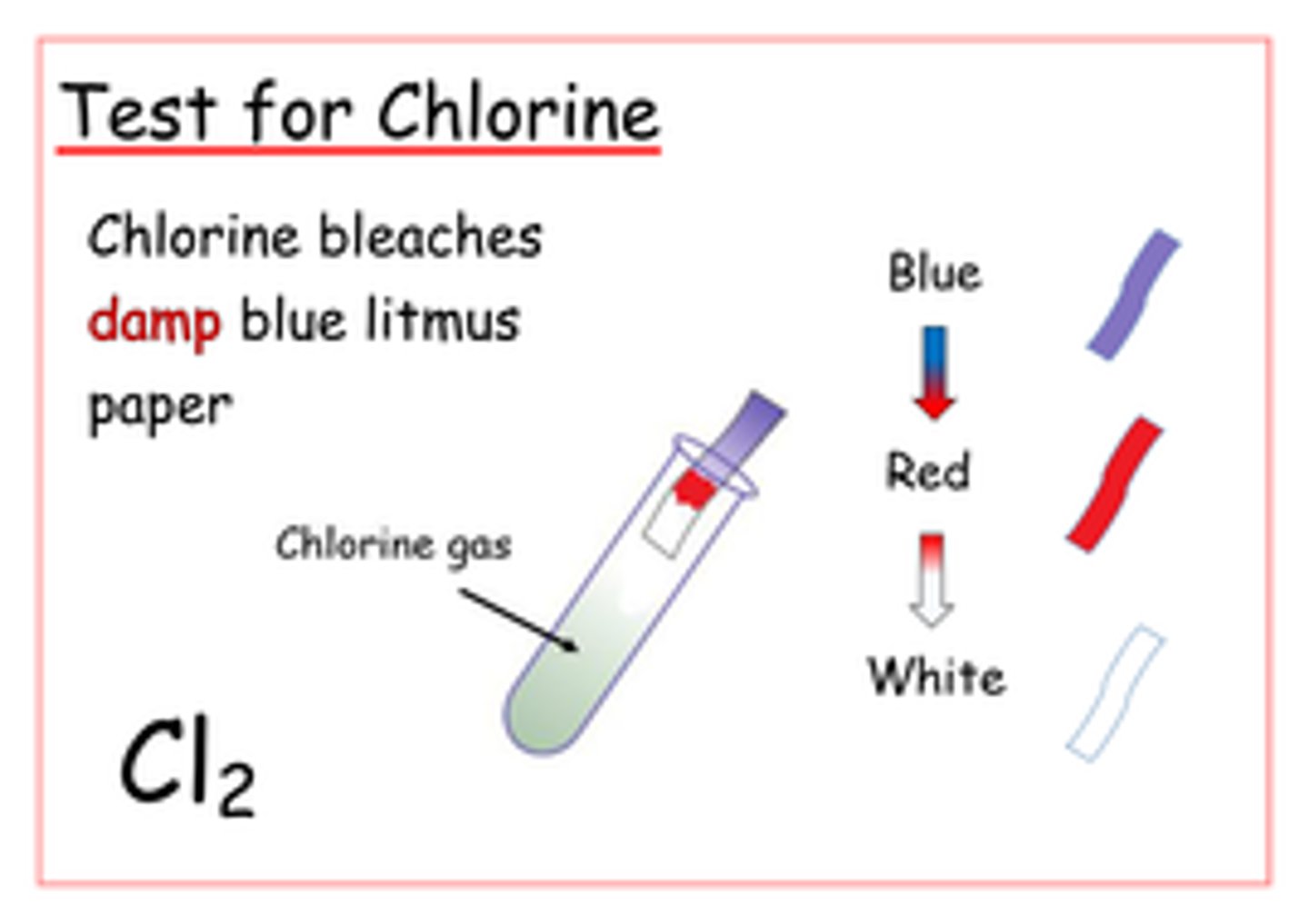
Chlorine Test
damp litmus paper -> put at mouth of test tube -> bleaches red side -> bleached blue side after turning it red
Ammonia test
damp RED litmus OR UI at mouth of test tube -> ammonia turns it blue
Li+ colour
red

Na+ colour
yellow

K+ color
lilac

Ca2+ colour
orange-red

Cu2+ colour
blue-green

Cu2+ + NaOH ->
Blue precipitate

Fe2+ + NaOH
Green precipitate (orange at top)

Fe3+ NaOH
Orange-brown precipitate

NH4+ + NaOH
No precipitate, ammonia gas produced
carbonates (C03^-2) test
1) Add dilute HCl to SOLID CO2-3
2) look for fizzing
3) If sample fizzes, repeat test but use delivery tube to bubble through limewater
The limewater should go cloudy
Sulfates (SO4 2-) test
1) Magnesium SULFATE solution in test tube
2) Add dilute HCl (to remove other anions)
3) Add BARIUM CHLORIDE solution dropwise with pipette
4) White precipitate = sulfate ions
White precipitate formed

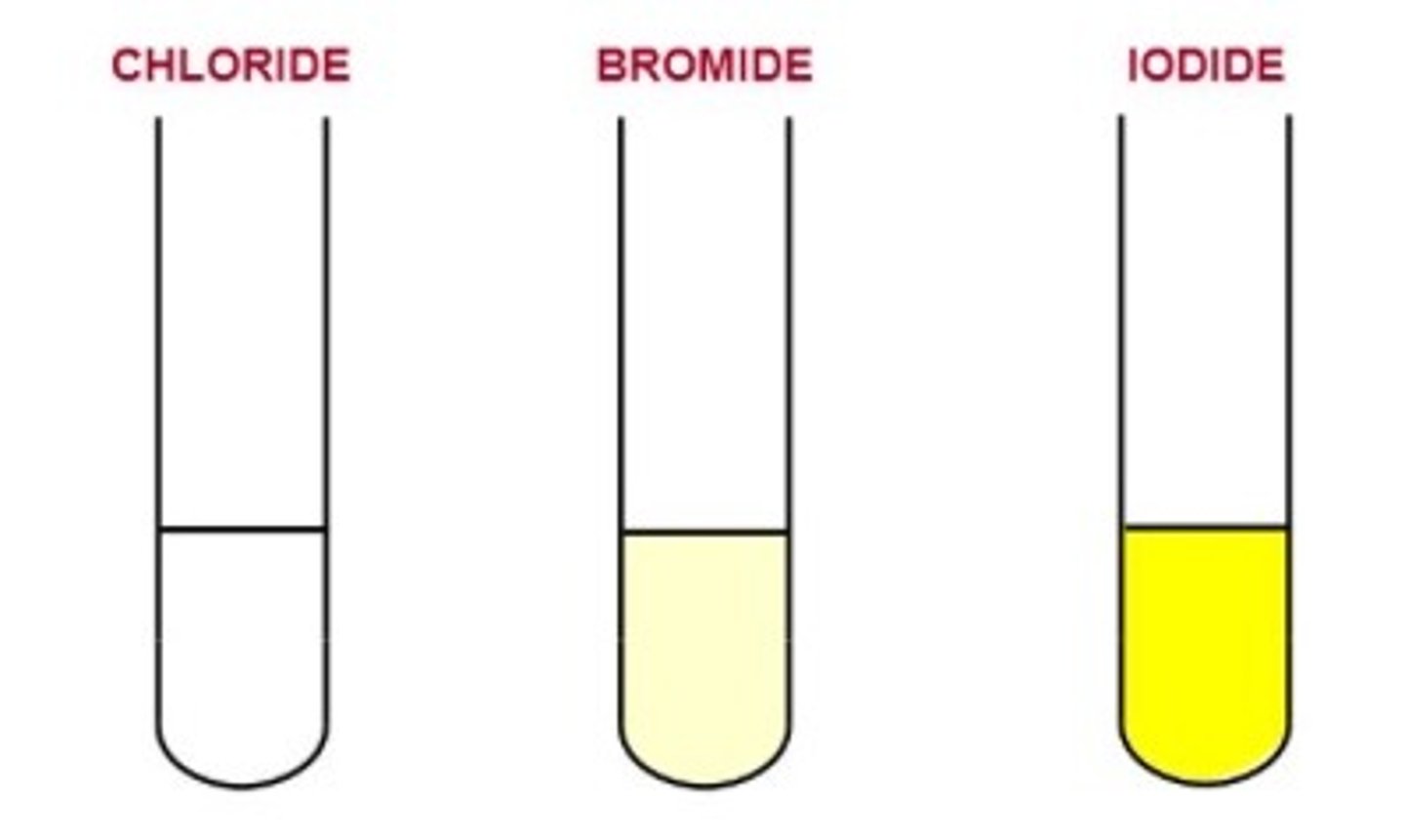
General test for halides
1) Add 1 pipette squirt of DILUTE NITRIC ACID to test tube of halide solution
2) Add silver nitrate solution
3) observe precipitate colour
Cl- halide precipitate
White
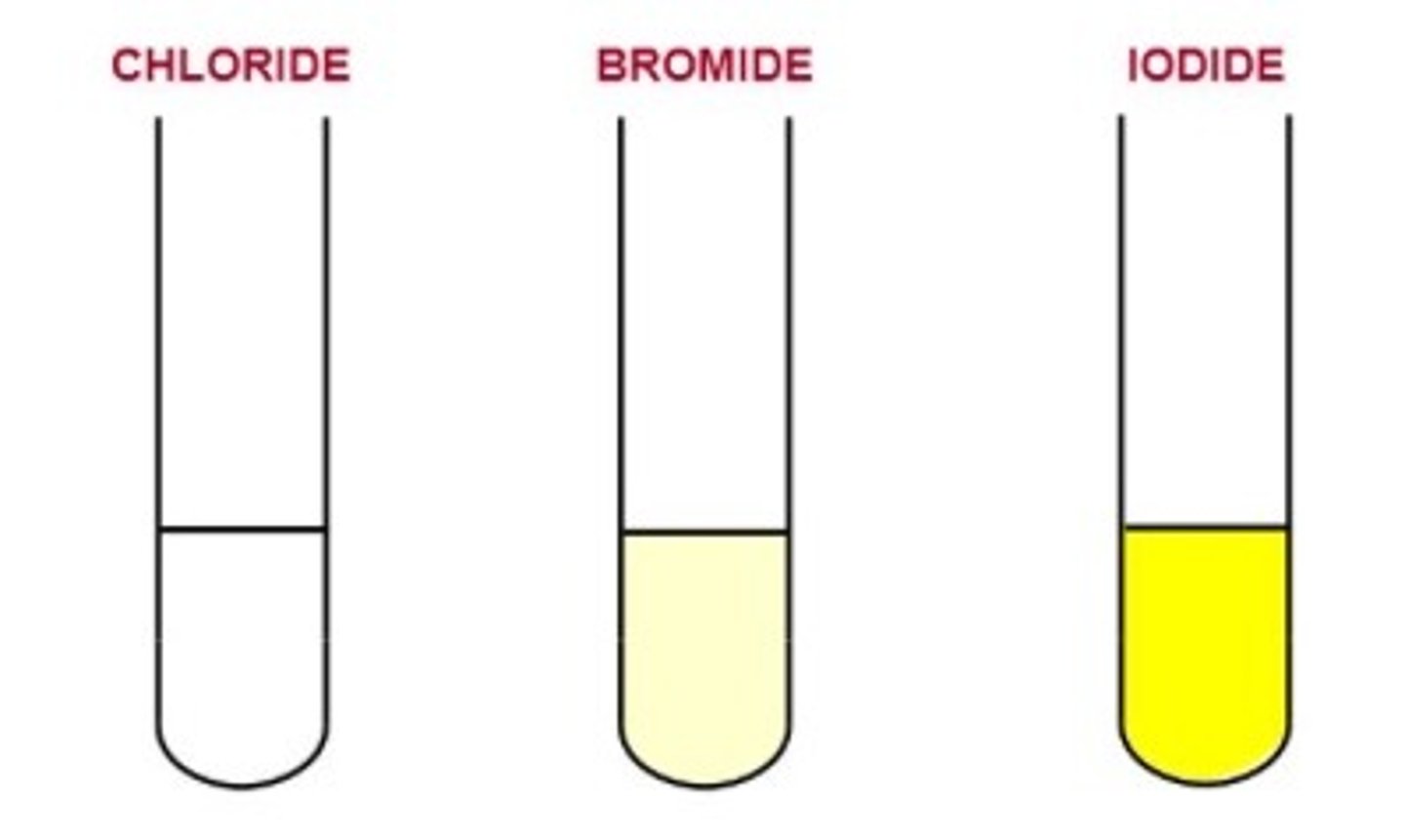
Br- halide precipitate
Cream
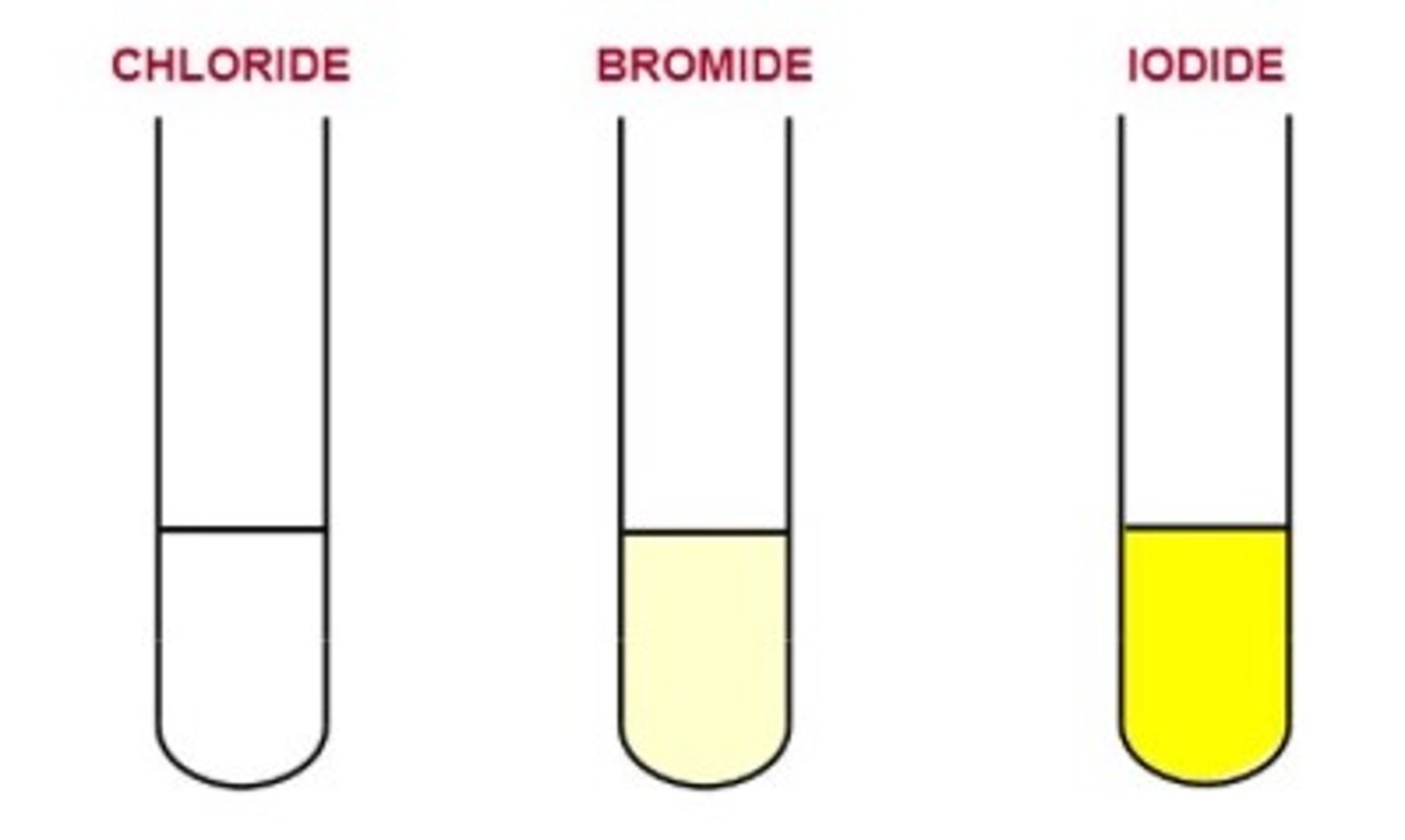
I- halide precipitate
Yellow