BMB Signalling
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
Why is cell signalling necessary?
To allow organisms to interact with their environment successfully.
To convey info around multicellular organism.
Facilitate coordination
What are the basic principles of cell signalling?
· A specific receptor for the stimulus in question.
· A transduction mechanism to transfer information from outside to inside the cell. Often this will include an amplification step.
· A signalling pathway, such as an enzyme which generates a second messenger, further amplifying the incoming signal.
· An effector protein, such as a protein kinase, able to deliver information to the final target system. Again, this will usually incorporate amplification.
· Mechanisms for terminating the signal e.g. down-regulation of the receptor and degradation pathways for the second messenger.
What are the three types of receptor protein?
enzyme coupled receptors
G protein coupled receptors
ion channels
more than one receptor type exists for a single ligand each linked to a different signalling mechanism. not linear on off, affected by amplification steps and feedback loops
Name some types of cascade.
MAP kinase cascades which lead to protein phosphorylation
Guanyly cyclases which make cGMP
Adenylate cyclases which make cAMP
Phospholipase C makes DAG and IP3
Calcium signalling via calmodulin/
Convergence and crosstalk of these pathways increases sensitivity
What are the functions of gap junctions within signalling cascades?
Interaction with neighbouring cells, makes a pore like structure. no receptors needed, alpha helical transmembrane structure, signalling molecules pass. If cells are touching, signalling may occur through pores in the membranes, such as gap junctions in animals or plasmodesmata in plants
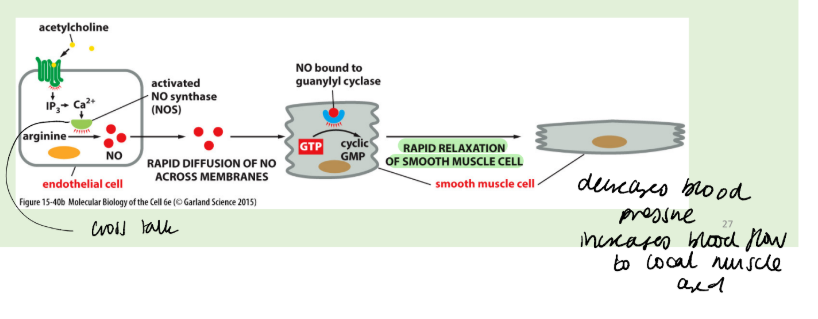
How does nitric oxide provide a signalling response?
Nervous, immune and circulatory systems.
Action is local since it is extremely unstable
Rapidly converted to nitrates and nitrites.
NO generated by nitric oxide synthase from metabolism of L-arginine.
NOS is a Ca2+/CaM-dependent enzyme, so that NO synthesis may be part of the amplification of another signalling event. A primary ligand may lead to a calcium signal in the cell, causing a set of effects which include NO synthesis. NO may then act intracellularly, or locally by diffusion into nearby cells.
The availability of NO limits the activity of GC. The GCs are soluble and heterodimeric with a haem group.
The binding of NO to haem restores its planar structure pulling the coordinating Histidine105 residue of GC towards the haem ring, thus activating the enzyme to produce cGMP. cGMP activates protein kinase G.
NO may be produced locally, e.g. by vascular endothelium, to target underlying vascular smooth muscle cells and maintain the vasculature in a relaxed state and to inhibit the propensity of circulating platelets to aggregate. Alternatively, the same cell may both synthesise and respond to NO (autocrine signalling)
Phosphodiesterases convert cGMP to GMP. This rapid degradation of cGMP constantly balances its production.
What are some drugs that target NO signalling?
NOS inhibitors = prevent anaphylatic shock by stopping the drop in blood pressure, prevent action of guanyl cyclase and production of cGMP.
Structural analogues of arginine block NOS activity.
Vasodilators nitroglycerine and nitroprusside act by release NO to activate GC = vasodilation,
Viagra maintains cGMP by blocking cGMP PDE. Supposed to lower blood pressure, viagra binds to substrate cleft of PDE 5, blocks cyclic AMP PDEs but lower potency.
The effect of Viagra is to enhance existing stimuli from the nervous system. Hence it has little benefit in dilating coronary arteries or in tonically lowering blood pressure.
What are some examples of small hydrophobic molecules that are signalling molecules? What are the receptors for these molecules?
Steroid hormones:
Cortisol, produced by adrenal glands in response to stress.
Oestradiol and testosterone affect sexual development and function.
Thyroid hormone is synthesized from tyrosine in the thyroid gland: it is important in development and metabolism.
Thyroid hormones
Thyroxine is important in development and metabolism.
Retinoids
Retinoic acid is made from vitamin A and is a local mediator in vertebrate development.
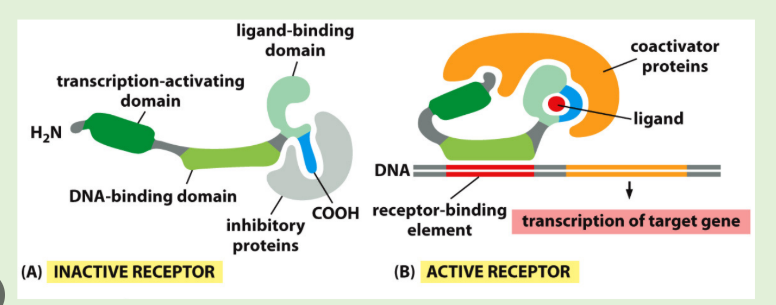
Nuclear receptor superfamily, transcription factors that have domains for ligand binding, DNA binding and transcriptional activation
Some steroid receptors are inactive in the absence of hormone due to other binding partners e.g. Glucocorticoid receptor is bound to Hsp90 in the absence of hormone. Glucocorticoid binding displaces Hsp90 and leads to binding of regulatory DNA sequences. Nuclear receptors can alsobind coactivators to help upregulate gene expression. For example, coactivators with histone cetyltransferase activity make downstream DNA more accessible to transcription.
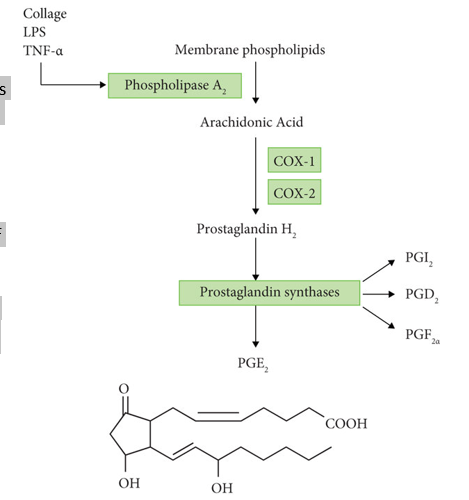
How do eicosanoids work as signalling molecules?
Eicosanoids are lipid-derived signalling molecules. They include the prostaglandins (PGs), thromboxanes (TXs). leukotrienes (LKs) and lipoxins (LXs). Prostaglandins and thromboxanes are produced by the cyclo-oxygenase pathway. Hydrolysis of membrane lipids to produce arachidonic acid is mediated predominantly by phospholipase A2. This important enzyme undergoes multiple levels of regulation. Arachidonic acid is converted to PGG2 by cyclo-oxygenase (COX) and thence rapidly to PGH2, the precursor of PGs and TXs.
COX is the target for several anti-inflammatory drugs, including aspirin, which acetylates and inactivates COX irreversibly, and non-steroidal anti-inflammatory drugs such as ibuprofen. Inhibiting synthesis of the prostaglandins reduces inflammation and pain.
How do water soluble hormones act as signalling molecules?
Adrenalin and Noradrenalin prepare the body for action. They act via a and b-adrenergic receptors, which respond differently and often oppositely.
Peptide hormones e.g. Glucagon, insulin and pituitary gland hormones (such as growth hormone, follicle-stimulating hormone and prolactin).
How do neurotransmitters, growth factors and cytokines act as signalling molecules?
Neurotransmitters carry signals between neurons or from neurons to other types of target cells. Release of neurotransmitters is signalled by arrival of an action potential at the end of a neuron. The neurotransmitters then diffuse across the synaptic cleft and bind to receptors on the target cell surface.
Growth factors
Nerve growth factor (NGF) is a member of the neurotrophin family that regulate the development and survival of neurons.
Epidermal growth factor (EGF) stimulates cell proliferation.
Platelet-derived growth factor (PDGF) stimulates proliferation of fibroblasts, contributing to regrowth of the damaged tissue.
HGF produced in the pituitary gland. excess causes pituitary tumours, deficiency leads to growth failure, artificial hgh used as doping agents.
Cytokines are peptides, proteins or glycoproteins that are secreted by numerous cells of the immune system, and some other cells, such as glial cell of the nervous system. They are usually pro-inflammatory and important in host immune responses.
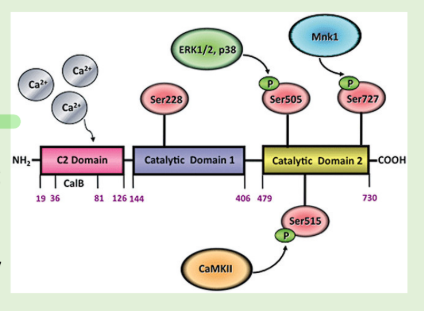
How does phospholipase A2 work in signalling?
Binding of Ca2+ to the C2 domain promotes the translocation of PLA2 from cytosol to membrane
• PLA2 is phosphorylated on key serine residues by MAPKs (ERK1/ERK2 and
p38), Ca2+/CaM-dependent protein kinase II (CaMKII), or MAPK-interacting kinase (Mnk1)
• Ser228 is in the first catalytic domain and is critical for the enzymatic activity
of PLA
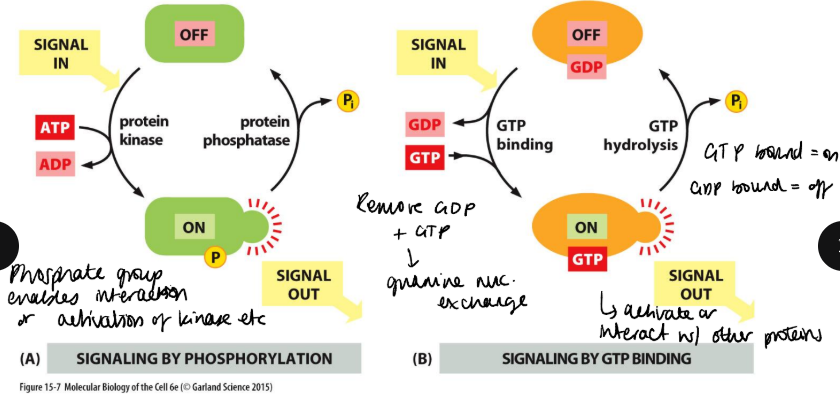
How do switch proteins work and what is phosphorylated?
Proteins that switch between an active/inactive often found in signalling pathways. Switch proteins can by activated by phosphorylation by protein kinases, which is removed by protein phosphatases. G proteins are active when bound to GTP and inactive following hydrolysis of GTP to GDP.
There are three amino acids with hydroxyl groups, which can be phosphorylated: serine, threonine and tyrosine. Kinases have 3 specificities.
1. Ser/Thr dual specificity kinases account for ~ 99% of cellular protein
phosphorylation.
2. Tyrosine kinases (TKs) are key elements in many signalling pathways.
3. Dual Thr/Tyr kinases are quite unusual and have very specific targets.
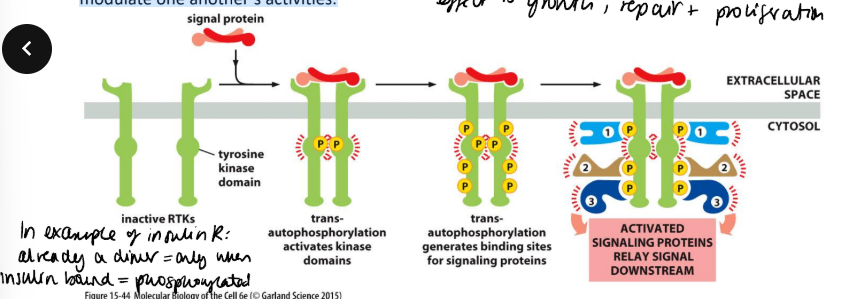
How do receptor tyrosine kinases detect and transmit signalling?
they have an N-terminal extracellular ligand-binding domain, a single transmembrane α helix and a cytosolic C-terminal domain with protein-tyrosine kinase activity.
Stimulation is two stage, receptor dimerisation and autophosphorylation. In addition, these phosphotyrosine residues act as a template to bind other signalling proteins, via their SH2 domains.
A single receptor dock several different SH2-containing proteins. This allows the receptor to form a signalling complex, allowing various diverse molecules to co-localise, to interact and to modulate one another’s activities.
Is dimerisation sufficient? no insulin receptor exists as dimers and is only active once occupied by insulin - due to conformational change which leads to autophosphorylation.
Some need adaptor proteins to hold large protein complexes together. they contain specialised domains which act as docking sites. Downstream signalling may occur via an adaptor protein and a Ras-activating protein, resulting in the activation of the small G protein Ras. In turn Ras can activate MAP kinase cascades.
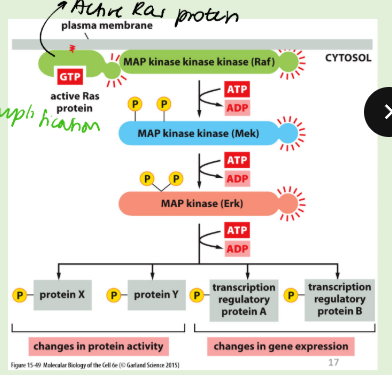
How do map kinase cascades work?
The MAP kinase pathway is a cascade of protein kinases that
amplifies the signal
MAP kinases are serine/threonine kinases with a Thr-X-Tyr activation
motif
The Thr and Tyr resides are phosphorylated by a MAP kinase kinase
These enzymes form a phosphorylation cascade that amplifies the signal.
Downstream lie more kinases (MAPKAPS), and transcription factors, which are activated by phosphorylation.
Highly conserved and found in all eukaryotic cells
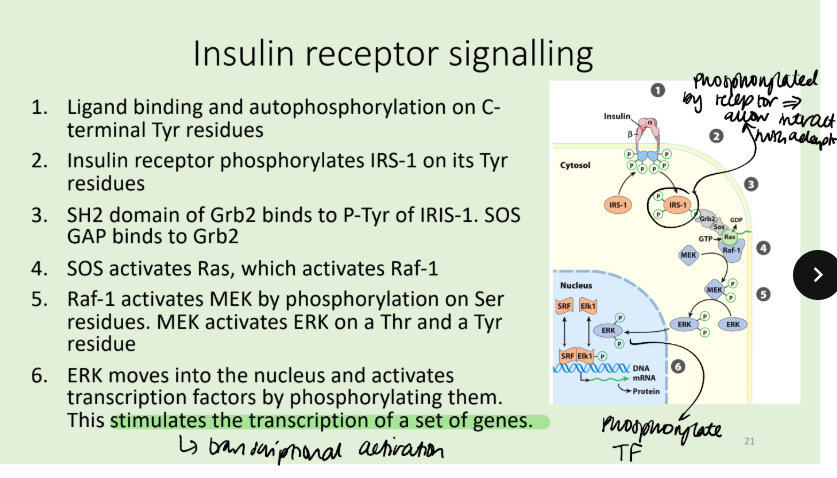
What is the mechanism for insulin receptor signalling?
The insulin receptor recruits a single species, Insulin-Receptor Substrate 1 (IRS-1), which is extensively phosphorylated and acts as a scaffold on which other downstream signalling proteins can cluster.
The insulin receptor protein (IR) is a dimer of αβ monomers. Binding of insulin between two subunits of the dimer activates the Tyr kinase activity of IR and each β phosphorylates three critical Tyr residues near the carboxyl terminus of the other β. autophosphorylation opens up the active site so that the enzyme can phosphorylate Tyr residues of other target proteins.
Genetic mutations to receptor = more serious than diabetes
What are indirect protein tyrosine kinases?
They are similar to receptor protein tyrosine kinases, but the cytosolic domains have no catalytic activity e.g. cytokine receptors. Ligand binding induces dimerization of receptors and cross- phosphorylation of associated non-receptor protein tyrosine kinases
What are the actions of insulin?
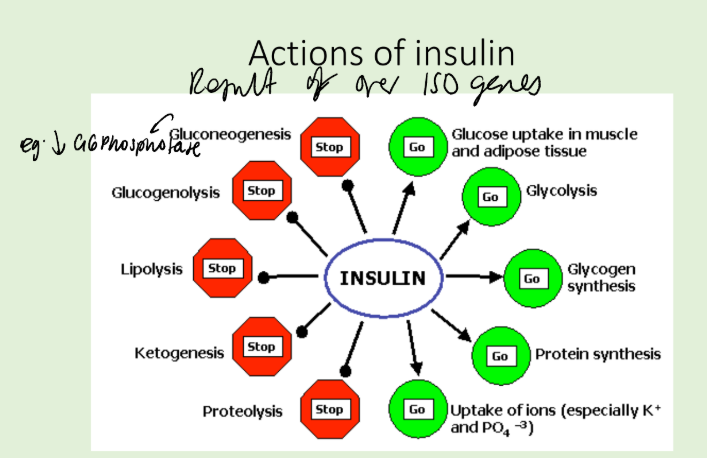
How are tyrosine kinases affected in cancer?
Due to roles in proliferation and survival, dysregulated RTK signalling impacts cancer
RTKs become aberrantly activated by overexpression, activating mutations or oncogenic function.
Cancer can induce reeptor overexpression, activation mutations or oncogenic fusions.
What are the proteins responsible for turning off the signalling cascades?
Three groups of phosphatases exist (with unrelated catalytic sites), non-specific, phosphoserine/threonine-specific (the PPases) and phosphotyrosine-specific enzymes (PTPases).
The first group (acid and alkaline phosphatases) can hydrolyse phosphomonoester bonds in both protein and non-proteinaceous substrates.
How do PPases and PTPases work?
PTPases: can be transmembrane receptors (e.g. CD45) or intracellular PTPases, which are activated by tyrosine phosphorylation.
There are four families of these multi domain proteins. Each contains at least one conserved phosphatase domain that has the 11-residue signature sequence I/VHCXAGXGRS/TG, the CX5R motif, which contains the enzymes catalytically essential Cys and Arg residues.
During the hydrolysis reaction the phosphoryl group is transferred from the tyrosyl residue on the substrate protein to the essential Cys on the enzyme forming a covalent Cys-phosphate intermediate that is subsequently hydrolysed.
There are 107 PTPases in the human genome, a similar number to the tyrosine kinases. However, it seems that the PTPases are not very specific, so the same PTPase can de-phosphorylate several related kinases.
PPases
There are two families of PPase (PPP AND PPM). A well characterized example is PP2A, which is involved in regulation of metabolism, DNA replication, transcription and development. It is a heterotrimer with scaffold (A), regulatory (B) and catalytic (C) subunits. Subunit A has 15 repeats of HEAT, in a horseshoe shaped arrangement; B= 8 HEAT like repeats.
How do bacteria target signalling pathways?
Many use a type three secretion system (T3SS) to deliver virulence proteins, These includes Yersinia pestis, the causative agent of bubonic plague and Shigella. Both bacteria deliver specific effectors that switch off immune signalling pathways.
Toll-like receptors activate MAPK pathways in response to PAMPs, eg LPS and flagellin from bacteria. These signalling pathways result in the increased expression of pro-inflammatory cytokines.
The Yersinia effector YopH is a T3SS effector that is a phosphatase. It has homology to eukaryotic PTPs. However, it is exceptionally active and seems to dephosphorylate many targets. It can break down signal transduction pathways in immune cells, therefore inhibiting the immune response.
The Shigella effector OspF specifically inhibits MAPK signalling as seen by block in Erk phosphorylation. However, mass spectrometry experiments determined it cleaves the C-OP bond of a threonine residue. since mass decreased of the protein when the toxin was added. Therefore, irreversible effect.
How do G protein coupled receptors generally work with reference to their structures?
7 alpha helices site of action of many drugs
medium affinity
2 discrete conformations resting and active, N terminus is extracellular C terminus is intracellular
ligand, recognises binding pocket, that lies quite deep within the cluster of transmembrane helices that form the receptor but can be formed by the external loops of the receptor. Ligand binding causes a conformational change from resting to active. This allows the binding of the corresponding heterotrimeric G protein to its cytoplasmic face.
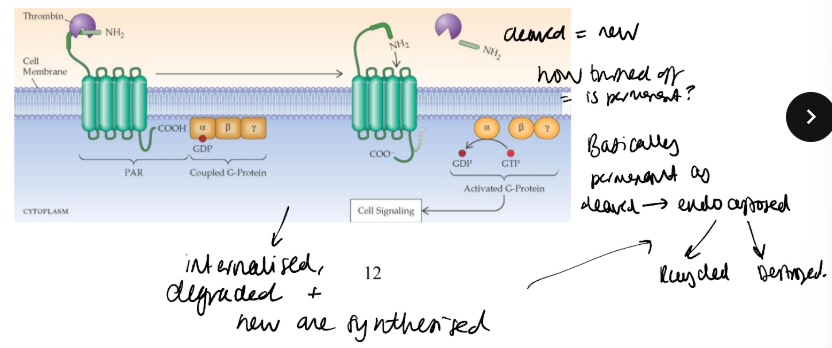
Describe the action of the thrombin PAR receptor and how its signalling cascade functions.
PAR1 is a receptor for thrombin, a serine protease important in platelet activation.
assumed that thrombin was a conventional ligand, occupying to activate.
cloning of receptor revealed a thrombin-cleavage site close to the N terminus, and functional studies showed that thrombin clipped off a short peptide revealing a new N-terminus with the sequence Ser-Phe-Leu-Leu-Arg-. This peptide binds to the receptor, activating it conventionally. The receptor is said to contain a tethered ligand.
Synthetic peptides of this sequence, Ser-Phe-Leu-Leu-Arg, known as thrombin receptor activatory peptide or TRAP, stimulate the receptor without cleavage.
Once cleaved permenantly activated so endocytosed degraded or recycled.
What do studies on the adrenergic A2 receptor show about the receptor mechanism?
A2 leads to decrease in AC activity.
Ligand binding initiates conformational change to the intracellular
domains of the molecule. Mutational studies identified clusters of amino acids close to the inner surface of the plasma membrane as crucial to signal transduction, and the production of chimeric receptors in which the 5th and 6th helices and their connecting 3rd loop have been substituted, can redirect the receptor to a different G protein.
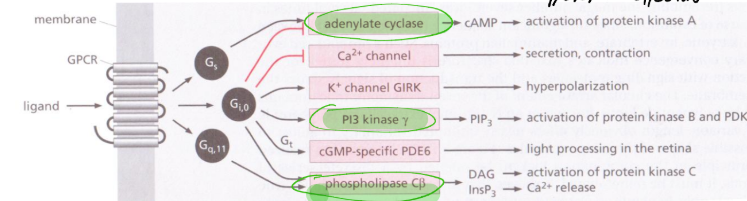
What is the structure of heterotrimer G proteins like and what are the different kinds functions?
Heterotrimeric G proteins associate with the plasma membrane, and comprise 3 subunits, α, β and γ,
The α and γ subunits are lipidated,which attaches them to the plasma membrane. The β and γ subunits are difficult to separate and operate as a single unit. Binding to an active receptor leads to exchange of GDP for GTP on the α subunit. The α subunit then dissociates from the rest of the G-protein. Both the GTP-bound α subunit and the βγ complex can bind downstream effector proteins.
The different categories of G protein couple to different effectors and this can be even seen in different adrenergic receptors.
The first signalling G protein discovered was Gs the AC stimulating one. through studies in a lymphoma cell line that lacked it.
Describe the structure of AC and its role with cAMP and what it does downstream.
The mammalian adenylate cyclases form a family of 9 different enzymes. They possess two domains (M1 and M2) each of 6 helices, separated by a cytoplasmic loop (C1) of about 400 amino acids, which, comprises the catalytic and regulatory sites of the enzyme. It converts ATP to cAMP.
Different types of AC are found in different bodiy parts and have different stimulators and inhibitiors including the Bg subunit variably acting to suppress or increase the AC workflow.
Most effects of cyclic AMP are the result of increased activity of
PKA. PKA exists as a tetramer of 2 regulatory and catalytic
subunits, which dissociates upon binding of two cAMP to each regulatory molecule.
A-kinase anchor proteins can bind both to the regulatory PKA, and to components of cytoskeleton or the membrane, thereby tethering the enzyme complex to a particular subcellular compartment
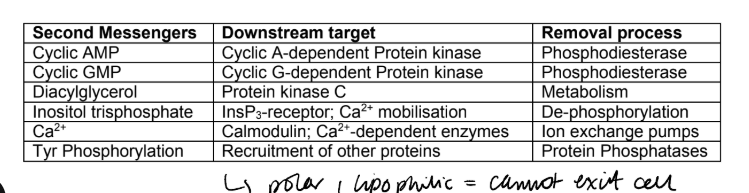
What are the downstream effects and removal processes for cAMP, cGMP, DAG, IP3, Ca2+, pTyr?
some second messengers are highly polar, water-soluble molecules and have no means of crossing the cell membrane, whereas others are highly lipophilic, so that they do not diffuse away from the membrane where generated.
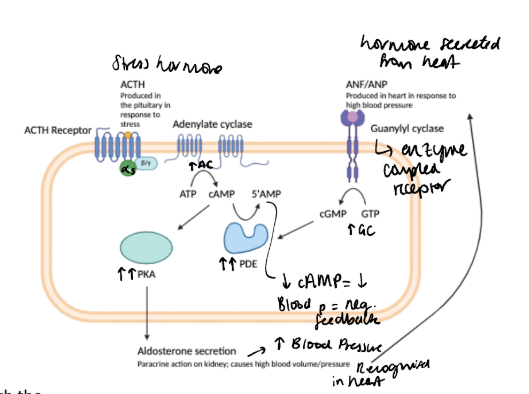
How do phosphodiesterases work to breakdown cAMP in the ACTH pathway? example of negative feedback loop
adrenocorticotrophic hormone (pituitary) stimulates aldosterone production by adrenocortical cells via a cAMP-dependent pathway in the adrenal. This leads to an increase in blood volume. To limit the rise in blood pressure, atrial natriuretic factor is synthesised in the heart atria and leads to cGMP generation through activation of guanylate cyclase. In turn, cyclic GMP binds and activates PDE II, preventing the activation of PKA and turning off aldosterone production in a negative feedback loop.
How do G proteins lead to PI3K activation?
G-proteins can also activate phospholipase C leading to the production of IP3 and DAG. IP3 releases Ca2+ from the ER, while DAG activates PKC by recruiting it to the plasma membrane.
PI3K phosphorylates the 3 position hydroxyl group of the inositol ring of phosphatidylinositol. It exists as a dimer, with regulatory subunit (p85) and a catalytic subunit (p110).Both are modular proteins and specific sites allow them to interact with one another and signalling molecules. The modular structure of PI3-Kinase allows it to serve an adaptor too.
As well as activation from GPCRs (e.g. the thrombin receptor in platelets), it can be activated by intracellular tyrosine kinases, which interact with either SH2 or SH3 domains of p85, and Ras, which interacts directly with p110.
Signalling proteins containing PH domains or other phosphoinositide-binding domains, are recruited to various cellular membranes by interaction with the phosphoinositides produced by PI3Ks. The relatively low abundance in membrane make them useful,signalling molecules. Plus, inositol has lots of phosphorylation sites and different combinations means different effectors.
Effectors lying downstream from PI3-Kinase include the small GTP-binding protein Rac, some PKC and the P70 ribosomal S6 kinase. Many of the consequences of these actions are related to cytoskeletal function, including cell motility, vesicle trafficking and chemotaxis. Other effects less obviously mediated by the cytoskeleton that require PI-3 K activity include DNA synthesis and cell survival signalling.
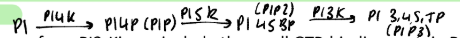
What are the diffferent proteins that a, b and g subunit of g proteins can activate or regulate or antagonise?
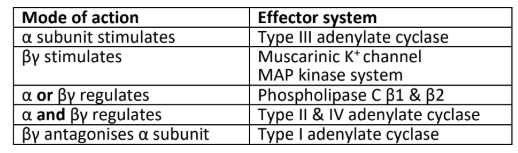
How are GPCRs involved in yeast mating?
The budding yeast Saccharomyces cerevisiae reproduces both asexually and sexually through mating and sporulation. The mating process occurs when two haploid yeast cells of opposite mating types (a and α) fuse to form a diploid cell (a/α). For this to occur each mating type secretes a pheromone which binds to a GPCR on the opposite mating type. α-factor binds the GPCR Ste2, which activates a heterotrimeric G protein. The Gby subunit recruits the MAPK adaptor Ste5 and the small G protein Cdc42, which activates the MAPKKK. This MAP kinase cascade results in the activation of pheromone response elements that changes in expression resulting in morphological changes associated with mating.
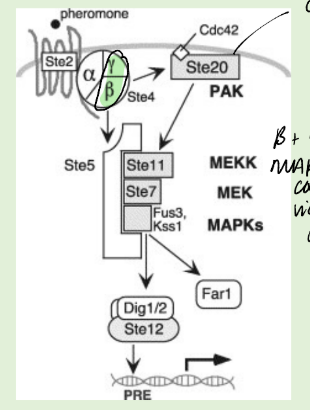
Draw the PLC signalling path out.
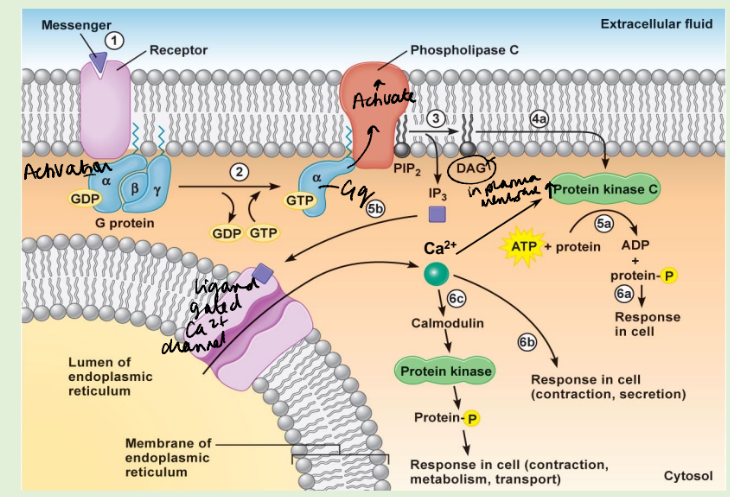
Describe how GPCRs can be heterologously desensitised?
phosphorylation of Ser residue in the C-terminal tail of the receptor, as a consequence of PKA activity. This blocks the capacity of the receptor to bind to Gs, but allows interaction with Gi, contributing to signal termination.
Any stimulus that activates AC will have this result, it is known as heterologous desensitisation – one ligand desensitizes the cell to the action of a second ligand.
A further effect of cAMP-dependent phosphorylation is to switch receptor specificity to a different G protein, in this case, Gi. Thus heterologous desensitization of b-AR turns OFF the activatory pathway and turns ON the inhibitory pathway.
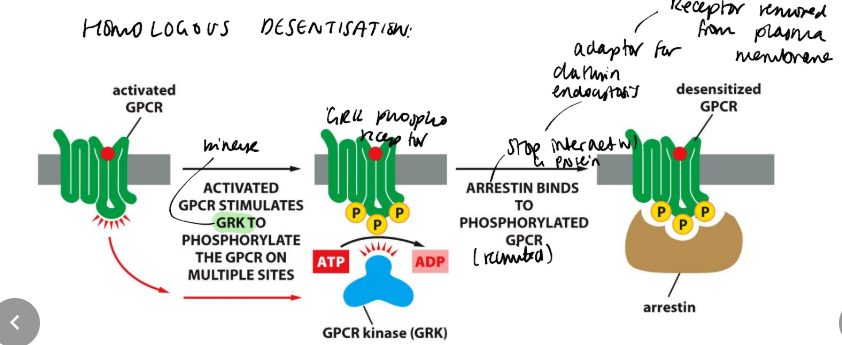
Describe how GPCRs can be homologously desensitised?
adrenergic receptor kinase (bARK), phosphorylates a group of Ser/Thr residues in the C-terminus of the receptor. These sites in the resting receptor are cryptic, i.e. not accessible, but become accessible in the activated receptor. Phosphorylation allows the receptor to recruit an inhibitory molecule, b-arrestin, which blocks signal transmission through the bAR only. Hence the action of bAR causes homologous desensitization. bARK also assists in the internalization and down-regulation of the receptor.
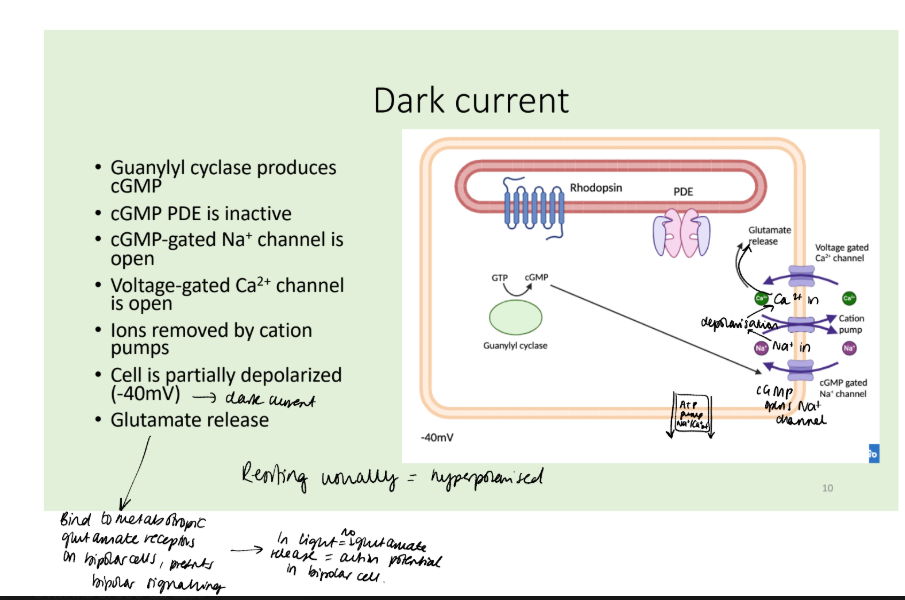
Describe the signalling pathway of rhodopsin and vision in the dark.
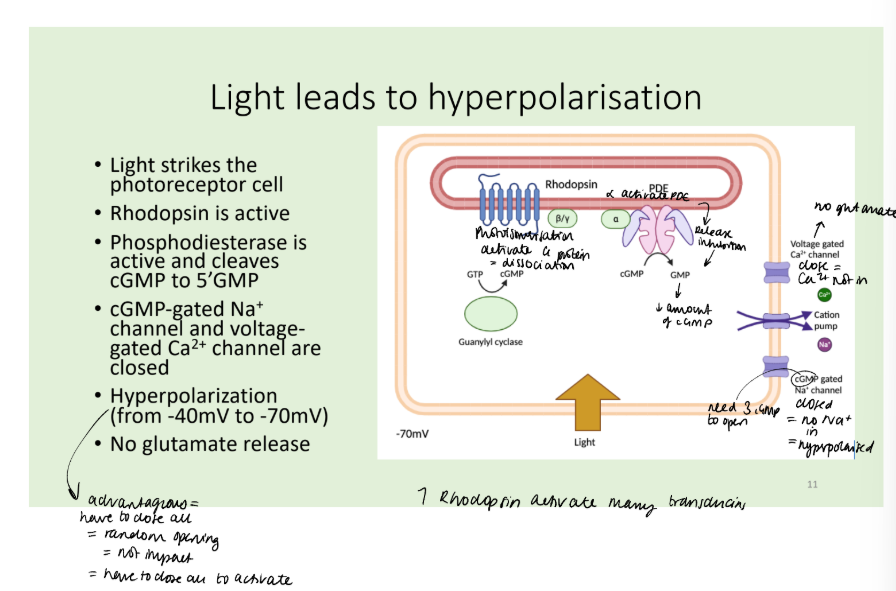
Describe the effect of light on the rhodopsin pathway.
Describe how the eye adapt to high light levels?
Guanylate cyclase is inhibited by Ca2+, so its activity rises as [Ca2+]i falls, helping to generate cGMP to return the channel to an open state. This effect is mediated by guanylate cyclase activating protein (GCAP), a protein that binds Ca2+ in its inactive state and stimulates guanylate cyclase in the absence of Ca2+.
Thus, cGMP promotes Ca2+ entry but Ca2+ inhibits cGMP formation and the two processes establish an equilibrium. This process is known as adaptation and allows the eye to function over a very wide range of ambient light levels.
This steady state is perturbed by altered PDE activity through light stimulation or by desensitisation of Rhodopsin and other signal terminating processes.
![<p>Guanylate cyclase is inhibited by Ca2+, so its activity rises as [Ca2+]i falls, helping to generate cGMP to return the channel to an open state. This effect is mediated by guanylate cyclase activating protein (GCAP), a protein that binds Ca2+ in its inactive state and stimulates guanylate cyclase in the absence of Ca2+.</p><p>Thus, cGMP promotes Ca2+ entry but Ca2+ inhibits cGMP formation and the two processes establish an equilibrium. This process is known as adaptation and allows the eye to function over a very wide range of ambient light levels.</p><p>This steady state is perturbed by altered PDE activity through light stimulation or by desensitisation of Rhodopsin and other signal terminating processes.</p>](https://knowt-user-attachments.s3.amazonaws.com/ce93eb00-e757-4e02-abf5-847bedee78b9.png)
How are signals terminated in rod cells?
The trans-retinal-lysine bond is rapidly hydrolysed, uncoupling it from the receptor, after which its cis conformation is restored, and it is recycled.
Rhodopsin kinase rapidly phosphorylates C-terminal serine residues of the receptor, allowing it to bind a new regulatory species, arrestin, preventing it from binding Gt. (receptor-desensitisation.)
The GTPase activity of Gt restores it to the inactive, GDP-bound, state.
How are cone cells different in signalling to rod cells?
cones use Gt2 protein not Gt1 to link to cGMP PDE.
different photopsins.
What are the ways of measuring cAMP/cGMP production?
adding radioactive [α32P]ATP to membrane samples and detecting the conversion to [32P]cAMP. [32P]cAMP is separated using ion-exchange chromatography and the amount of [32P]cAMP in the sample analysed.
Radioimmunoassays use immobilised anti-cAMP bound to radioactive cAMP. A whole cell or tissue lysate sample is then added to the antibodies and the cAMP from the sample will compete with radioactive cAMP
Can use FRET to see where cAMP produced and interaction partners.
A range of compounds have been used to study the G-protein cycle. Non-hydrolysable analogues of GTP have been very useful as they lead to the a-subunit of the G proteins becoming locked active. Hence adenylate cyclase or other G protein-regulated pathways, can be stimulated without the intervention of hormone receptors.
![<ul><li><p>adding radioactive [α32P]ATP to membrane samples and detecting the conversion to [32P]cAMP. [32P]cAMP is separated using ion-exchange chromatography and the amount of [32P]cAMP in the sample analysed.</p><p>Radioimmunoassays use immobilised anti-cAMP bound to radioactive cAMP. A whole cell or tissue lysate sample is then added to the antibodies and the cAMP from the sample will compete with radioactive cAMP</p></li><li><p>Can use FRET to see where cAMP produced and interaction partners.</p></li><li><p>A range of compounds have been used to study the G-protein cycle. Non-hydrolysable analogues of GTP have been very useful as they lead to the a-subunit of the G proteins becoming locked active. Hence adenylate cyclase or other G protein-regulated pathways, can be stimulated without the intervention of hormone receptors.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/de9e3ded-01c2-493f-85c3-10952e02fc5c.png)
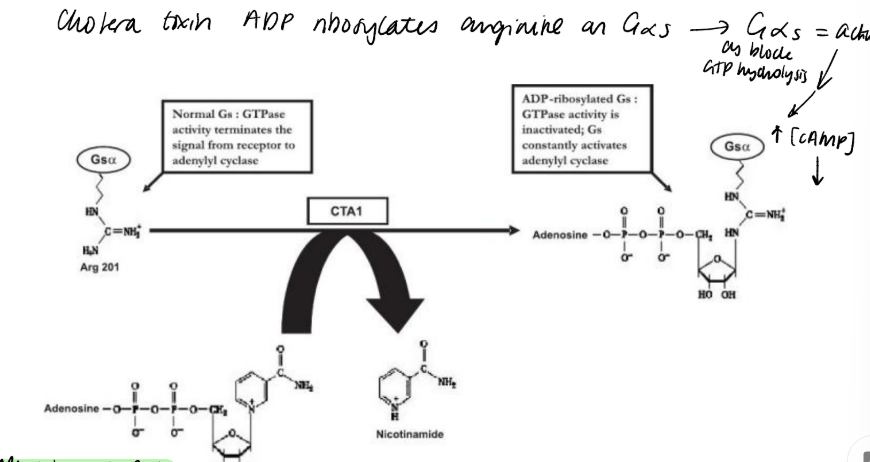
Describe the biochemical action of cholera toxin and pertussis toxin?
AB5 toxin enters via endocytosis, act upon Gs and Gi like proteins.
The target amino acid for cholera toxin is an Arg close to the GTP-binding site, preventing GTP hydrolysis, so becomes locked active.
The action of cholera toxin, has a similar outcome to the use of GppNHp or GTPS. At a physiological level, cholera toxin causes disastrous fluid loss from the gut lining.
Pertussis toxin transfers ADP-ribose to a cysteine residue in the C-terminus of Gi-like G proteins, blocking the capacity of Gi to interact with the receptor. Hence hormonal stimulation is not possible so bound inactive and NOT inhibiting AC so increases cAMP.
What are the different small GTPases and what do they do?
A second family of GTPases involved with signal transduction are the Ras superfamily of small GTPases. There are 5 subfamillies. Ras, Rho, Rab, Ran and Arf. They are involved in cellular processes as diverse as nuclear transport, cytoskeletal rearrangements, organelle transport, cell growth, differentiation and cell adhesion.
Small GTPases undergo the same cycle between GDP and GTP bound, inactive and active forms as heterotrimeric G proteins. However, Ras has low catalytic activity; it is not able to independently hydrolyse GTP to GDP sufficiently quickly. GTP hydrolysis is accelerated by GTPase activating proteins (GAPs), while GTP exchange is catalysed by guanine nucleotide exchange factors (GEFs).
How was Ras first discovered?
Identified as a viral oncogene, three isoforms.
Lipidation associates it to membrane
Leads to activation of transcription factors and regulation of gene expression.
70/90% pancreatic cancers and 20-50% lung cancers usually localised to a loop region in guanosine binding site.
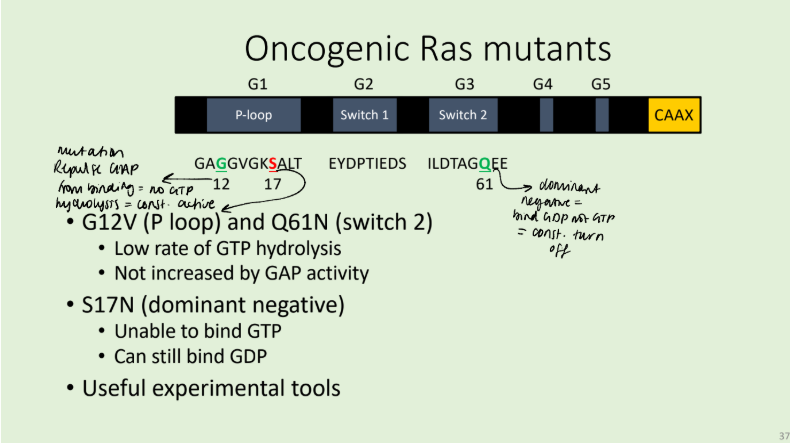
Describe the structure of Ras and the mechanism of its action.
five conserved regions involved in guanosine nucleotide binding (G1-5). The first three regions include P-loop (Phosphate binding loop, G1), and the switch regions 1 (G2) and 2 (G3). Switch 1 contains a conserved threonine at position 35, whilst switch 2 contains a glycine at
position 60. When the nucleotide-binding site is occupied by GTP, Thr35 and Gly60 are coordinated by the GTP -phosphate, producing a tense, active form of the protein.
When the phosphate is hydrolysed coordination releases and the molecule returns to a relaxed form. The effector domain, which interacts with downstream signalling proteins, such as Raf, encompasses switch 1 but is only in the correct conformation when GTP is bound.
In isolation, Ras is catalytically inactive. GTP hydrolysis requires the action of GAPs such as p120. GAPs accelerate GTP hydrolysis by introducing an arginine residue into the active site of Ras. This
arginine acts in conjunction with Lys16 of Ras (in G1) on the -phosphate of GTP, stabilising the transition state of GTP hydrolysis.
Insight into the interaction between Ras and RasGAPs explains how oncogenic mutations in Ras have their effect. Mutations at position 12 result in repulsion or steric hindrance. Thus, Ras is unable to hydrolyse GTP and will therefore remain in an active conformation.
In a similar manner to GAP activity, Guanosine exchange is achieved by GEFs inserting residues into the nucleotide-binding site, sterically and electrostatically inhibiting GDP binding. Once GDP is removed, GTP (present at a higher concentration in the cytosol) is able to occupy the Guanosine binding site.
What are the two types of ion channel and what are they used for?
Ion channels are important in many cells but especially in nerve and muscle.
Transport through ion channels is extremely rapid: more than a million ions per second.
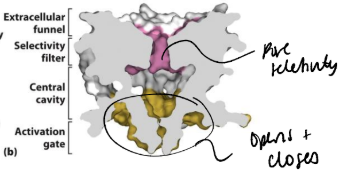
Ion channels can be highly selective.
Voltage-gated ion channels open in response to changes in electric potential across the plasma membrane.
Ligand-gated ion channels open in response to the binding of neurotransmitters or other signalling molecules.
Describe the mechanism of action of the voltage gated ion channels and how they are ion selective.
they are primarily made up of a single polypeptide with four homologous domains: 6 membrane-spanning helices. They do not have a large extracellular domain.
Voltage- gated K+ channels function in a similar way, with the exception that they are composed of four separate polypeptide chains, each comprising one domain.
One of the membrane-spanning helices, S4, is the voltage-sensing helix, which contains many positive charged amino acids. Depolarization causes the helix to move, inducing a conformational change that opens the channel.
Voltage-gated channels are more selective than ligand-gated ion channels. The Na+ pore is too small for K+ or larger. The K+ channels pore is lined with carbonyl oxygen from the polypeptide backbone of the pore helix re-entrant loop. They displace the water to which K+ is bound and the K+ ion passes through. Na+ is too small to interact and remains bound to water.
What is the structure and mechanism of ligand gated ion channels using AChR as an example?
multisubunit proteins. There are different families with 3, 4 or 5 subunits. The subunits in each of these families have a distinct structure. may be homo or heteromeric. have a large extracellular ligand binding domain.
The acetylcholine receptor (AChR) family is pentameric. Each has 4 transmembrane helices. The ligand is ATP.
Hydrophobic residues in the M2 helix of the AChR line the pore. Acetylcholine binds to the extracellular region in between two subunits. This causes a conformational change that is transduced to the membrane domain. The α-helices lining the pore ‘relax’ to remove the hydrophobic ‘girdle’ from the channel and allow ion flow.
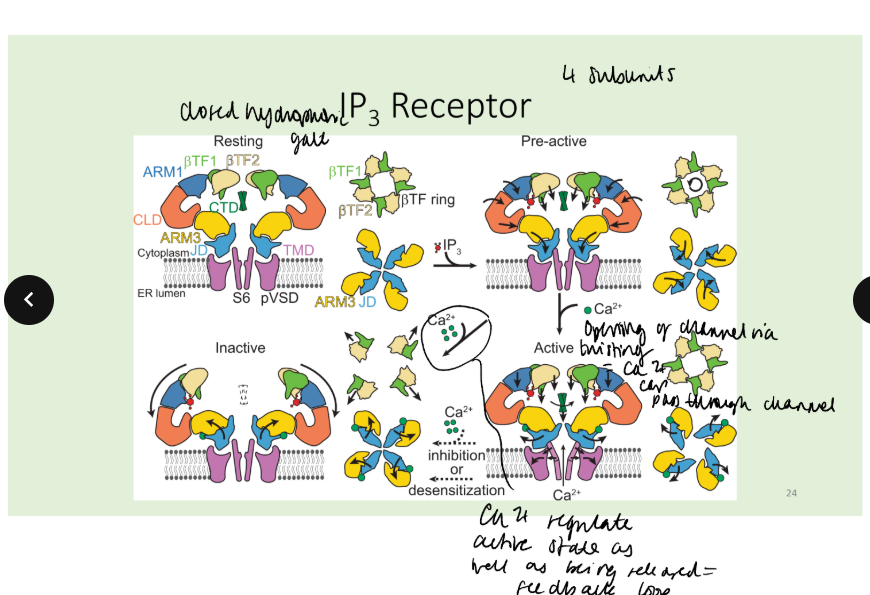
What are the two kinds of IP3 receptor and describe their mechanism.
There are two forms of phospholipase C: PLC- is stimulated by G proteins, PLC-γ is stimulated by tyrosine kinases. - convergence
In the resting state, the IP3 R sometimes opens briefly and spontaneously, this is an elemental event. This may trigger the opening of adjacent IP3 R, giving rise to a measurable Ca2+ transient – a puff or spark. This localized Ca2+ transient may lead to waves of Ca2, and if there is a sufficient external stimulus, to the global opening of IP3 receptors and to a sustained elevation of intracellular Ca2+.
What are the downstream effects of calcium, how does it work?
Calmodulin binds Ca2+ in its EF hand domains.
Conformational change occurs on Ca2+ binding and means CaM can interact with target proteins and regukate their funxtion.
In particular, CaM Kinase II acts as a memory device. Autophosphorylation in response to Ca2+/CaM. Remains active in the
absence of Ca2+. Frequency decoder of Ca2+ oscillations
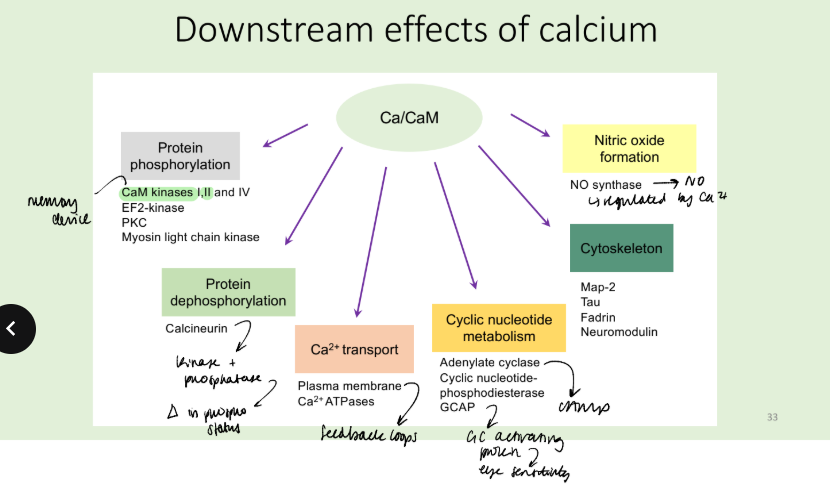
How is the Calcium signal terminated?
Ca2+ is exported from the cytoplasm to terminate the signal by three mechanisms. The ER is refilled using calcium pumps (SERCAs; sarco/endoplasmic reticulum Ca2+ ATPases); Na+/Ca2+ exchanger exports Ca2+ from the cell; high-affinity plasma membrane pumps (PMCAs) complete the process. The IP3 signal is terminated by further metabolism: phosphatases sequentially remove phosphate, yielding IP2 then IP, and then inositol.
The final stage of this can be blocked by Li+
PLC is also phosphorylated on Serine residues by both PKC and PKA. These events lower the activity of the enzyme.