DNA Profiling and Gene Therapy Overview
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
136 Terms
DNA Profiling
The process of determining an individual's DNA characteristics.
DNA Marker
A piece of DNA from a known location in the DNA molecule, which differs between people.
Polymerase Chain Reaction (PCR)
A method used to make millions of copies of DNA from a small sample.
Steps of PCR
1. Denaturation: DNA strands are heated (~95°C) to separate them. 2. Annealing: Primers attach to specific DNA sequences (~50-60°C). 3. Extension: DNA polymerase adds nucleotides to build new strands (~72°C).
Importance of PCR
Used to amplify tiny amounts of DNA (e.g., from crime scenes, fossils).
Gel Electrophoresis
The process of separating DNA, RNA and protein molecules through their different electrical charges and size.
Steps of Gel Electrophoresis
1. Gel (usually agarose) is spread out with an electrical charge running through it. 2. Restriction enzymes are used to cut DNA into specific pieces. 3. DNA is loaded into the 'wells' of the device. 4. Negative DNA moves towards the positive side of the device through the gel, shortest move faster. 5. DNA pieces are thus spread out, creating DNA bands. 6. Staining the gel and putting it under a UV light to see these bands.
DNA Ladder
A reference tool with known DNA fragment sizes to compare results.
Forensics & Criminal Investigations
DNA profiling is useful in solving crimes as it identifies the features of a person from a sample such as a hair or fingerprint left on the crime scene.
Limitations of Forensic DNA Profiling
1. Environmental factors can destroy DNA evidence (e.g., heat, sunlight, bacteria, and mold). 2. Identical twins share identical DNA. 3. DNA from relatives have similar DNA. 4. DNA cannot be used to determine the time the suspect was at the crime scene.
Paternity & Relationship Testing
The genetic factors of parents are equally inherited from both parents.
Chromosomes from Parents
Everyone has 23 chromosomes from each parent.
Alleles of STR
Everyone has two alleles of every STR in their genome.
Determining Parentage
A person's DNA profile should also be present in their mother and/or father's DNA profiles.
PCR for Paternity Tests
The PCR method is useful for DNA testing as it enables higher specificity in the region being selected for amplification.
PCR
PCR is the technique of amplifying specific strands of DNA samples, allowing the creation of enough material for thorough, accurate analysis.
Electrophoresis
Electrophoresis is used to separate DNA fragments by size using an electric current, helping visualize DNA fragments that can then be compared across samples.
Highly Accurate
DNA profiles are unique (except for identical twins).
Requires Small Samples
PCR allows analysis even from trace DNA.
Useful in Solving Crimes
Helps convict criminals or exonerate innocent people.
Medical & Ancestry Uses
Helps detect genetic disorders and study ancestry.
Errors in DNA Samples
Errors can occur if DNA samples are degraded.
Sample Contamination
Contamination can lead to inaccurate results if the DNA sample comes in contact with any form of contamination.
Privacy Concerns
DNA databases could be misused, raising significant privacy risks.
Ethical Issues
Family secrets (e.g., non-biological parents) may be revealed.
Cost & Time
Some analyses take time and may be expensive.
Discrimination and Stigmatization
Genetic information could be used to discriminate against individuals, affecting insurance and other areas.
Racial and Ethnic Profiling
DNA profiling has linked to racial and ethnic biases, particularly in forensic applications.
Accessibility
Access to DNA profiling technologies is biased towards wealthier individuals and communities.
Enhanced Sensitivity
PCR enables the analysis of DNA from tiny samples such as skin cells, hair, and blood traces.
Faster Results
PCR can amplify DNA within a matter of hours, significantly increasing the speed of DNA profiling.
Improved Accuracy
Electrophoresis allows the precise separation of DNA fragments through their charges.
Automation in Electrophoresis
Advancements in electrophoresis technology have resulted in automated systems, increasing speed and efficiency.
High Resolution
Modern electrophoresis systems provide high resolution in DNA fragment separation.
Disease Identification
Detect genetic disorders or mutations through DNA sequence variations.
Transplant Matching
Helps in organ donor compatibility.
What are the steps of DNA profiling
Sample Collection: DNA can be extracted from a variety of biological samples, such as blood, hair, saliva, or skin cells.
DNA Extraction: The DNA is extracted from the cells in the sample. This involves breaking open the cell and separating the DNA from other cellular components.
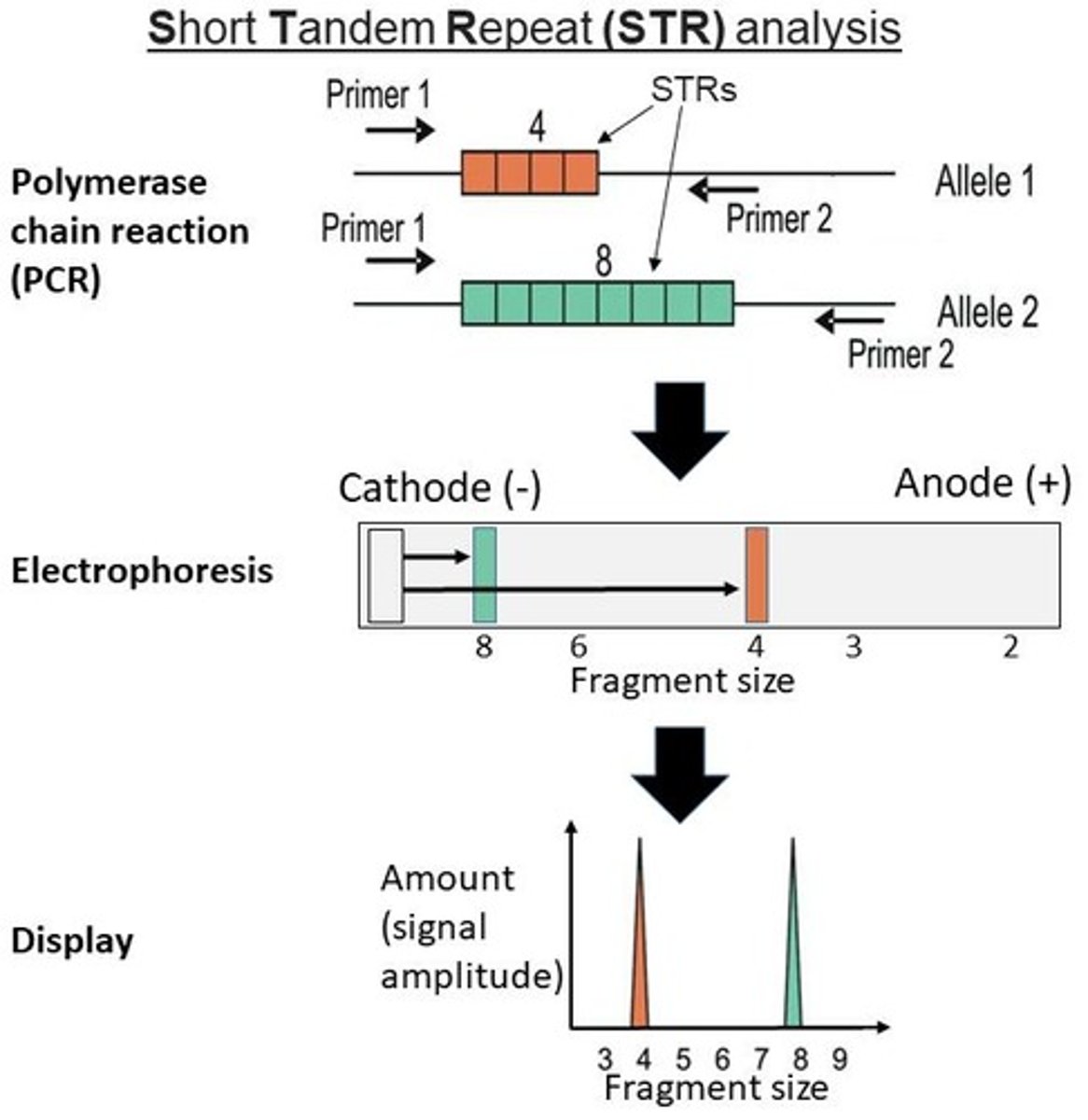
DNA Amplification (PCR): Specific regions of the DNA, called Short Tandem Repeats (STRs), which are known to vary among individuals, are selected for analysis. The polymerase chain reaction (PCR) is used to make many copies of these regions to ensure there is enough DNA for testing.
DNA Fragmentation: The amplified DNA regions are cut into smaller pieces using enzymes called restriction enzymes. These enzymes cut the DNA at specific sequences, creating fragments of varying lengths.
Gel Electrophoresis: The DNA fragments are then separated by size using gel electrophoresis. The gel is subjected to an electric field, which causes the DNA fragments to move through it. Smaller fragments move faster, while larger fragments move slower, creating a unique pattern of bands.
Visualization: The separated DNA fragments are visualized using a stain that binds to DNA. The resulting pattern of bands (a "DNA ladder") is unique for each individual, as the length and number of repeats in the STR regions differ from person to person.
Analysis: The band patterns from the gel electrophoresis are compared and analyzed. A DNA profile is created based on the specific locations and sizes of the STRs.
Case Study in Archaeology
In 2010, scientists successfully sequenced the Neanderthal genome using DNA extracted from Neanderthal bones discovered in the Neander Valley (Germany) and other sites across Europe. This groundbreaking work, led by the Max Planck Institute for Evolutionary Anthropology, involved extracting and analysing extremely fragmented and degraded DNA from Neanderthal remains.
Genetic Markers
STRs (Short Tandem Repeats) and SNPs (Single Nucleotide Polymorphisms) are examples of genetic markers unique to individuals.
Capillary Electrophoresis
Allows better resolution and more accurate results in DNA profiling, especially for complex samples.
Gene Therapy
An advanced medical technique that aims to treat or prevent diseases by modifying or replacing faulty genes within a person's cells.
Gene Replacement
Used when a disease is caused by a missing or defective gene; a healthy copy of the gene is inserted into the patient's cells.
Cystic Fibrosis Treatment
An example of gene replacement therapy where a working CFTR gene is delivered into lung cells to restore proper mucus production.
Gene Editing
The faulty gene is directly modified or repaired at the DNA level, using technologies like CRISPR-Cas9.
Sickle Cell Anaemia
An example of gene editing where CRISPR gene editing corrects the faulty hemoglobin gene in blood stem cells.
Gene Silencing
Used when a faulty gene is overactive or producing harmful proteins; small RNA molecules or modified DNA sequences are introduced to block or suppress a faulty gene.
Somatic Therapy
Gene therapy that affects only the patient.
Germline Therapy
Gene therapy that affects future generations; controversial.
Viral Vectors
Genetically modified viruses (e.g., adenovirus, lentivirus) that act as delivery vehicles for therapeutic genes.
Non-Viral Methods
Methods of gene delivery that do not use viruses, such as CRISPR and lipid nanoparticles.
Lipid Nanoparticles (LNPs)
Fat-based carriers that deliver genetic material, used in mRNA vaccines.
Electroporation
Uses an electrical pulse to temporarily open cell membranes, allowing genes to enter.
Direct Injection of DNA
Plasmid DNA is injected directly into tissues or bloodstream.
Ex Vivo Gene Therapy
Outside body delivery where the patient's cells are removed, genetically modified, and then returned into the body.
In Vivo Gene Therapy
In body gene therapy where the gene is delivered directly into the patient's body using a vector.
Identifying the Target Gene
The first step in gene therapy, using genetic testing to determine the gene and disease.
Gene Expression and Monitoring
Checking if the gene is functioning properly after delivery.
Long Term Follow Up
Monitoring the patient for potential adjustments after gene therapy.
CAR-T Cell Therapy
An advancement form of immunotherapy that genetically modifies a patient's T cells to recognize and destroy cancer cells.
Cytokine Release Syndrome
A severe immune reaction causing fever, inflammation, and organ damage as a side effect of CAR-T cell therapy.
Neurological Toxicity
Some patients experience confusion, seizures, or temporary brain swelling as a side effect of CAR-T cell therapy.
Haemophilia
Caused by mutations in the F8 or F9 genes, preventing the production of clotting factors and causing excessive bleeding.
Gene therapy
A viral vector delivers a functional F8 or F9 gene into the liver, enabling the body to produce clotting factors.
Hemgenix
A treatment for hemophilia B that reduces bleeding episodes by increasing clotting factor IX production.
Severe combined immunodeficiency (SCID)
A condition caused by mutations in genes like AdA that prevent the immune system from functioning.
ADA gene therapy
Viral vectors deliver a healthy ADA gene into the bone marrow stem cells to restore immune function.
Zolgensma
A gene therapy drug that treats spinal muscular atrophy.
Advantages of gene therapy
Potential cure for genetic diseases, long-lasting effects, and personalized medicine.
Traditional treatments
Manage symptoms of diseases rather than targeting the root cause.
High cost of gene therapy
Gene therapy treatments are extremely expensive due to complex manufacturing and specialized infrastructure.
Unintended mutations
Accidental targeting of the wrong part of the genome can lead to mutations or cancer.
Luxturna
The first gene therapy that costs over $400,000 per eye.
Designer Babies
Germline gene therapy where genes in eggs, sperm, or embryos are altered, leading to ethical debates.
Unintended genetic consequences
CRISPR and other gene editing technologies may cause off-target mutations, leading to unexpected side effects.
Leukemia from gene therapy trials
Some early gene therapy trials resulted in leukemia due to unintended DNA changes.
Costs and access to gene therapy
Gene therapy is extremely expensive, limiting access to wealthy individuals or countries.
Impact on Disability Communities
Gene therapy could reduce the number of people born with genetic conditions like Down syndrome or deafness.
Economic Impact of gene therapy
Successful gene therapy could reduce healthcare costs by eliminating lifelong treatments.
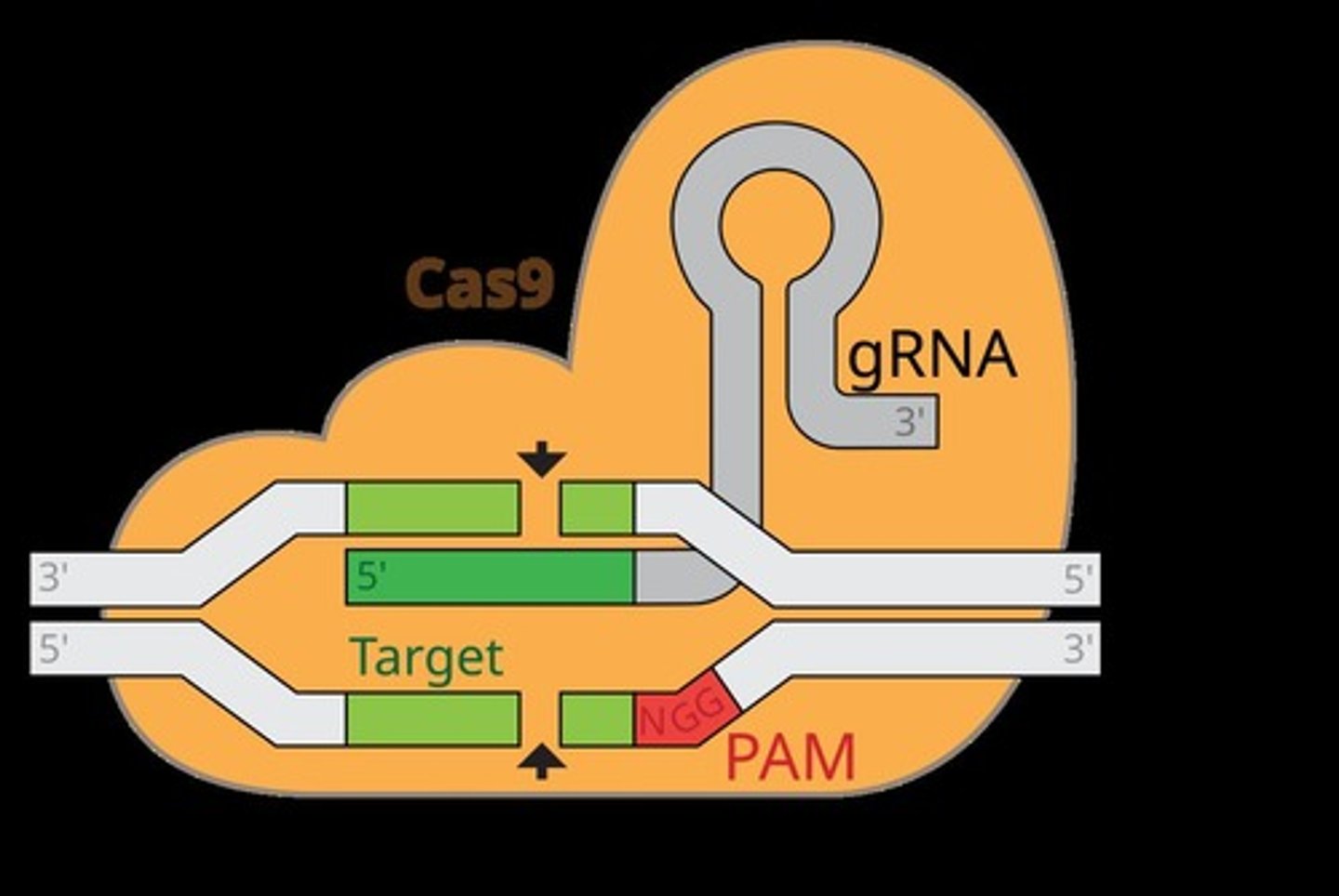
CRISPR-Cas9
A technology that allows faster, cheaper, and more precise gene editing.
Base editing
A method that can correct single-letter mutations without breaking the DNA strand.
Prime editing
An even more precise method of gene editing that reduces the risk of off-target effects.
Viral vectors
Designed to target specific tissues such as the liver, brain, or muscles, helping reduce the risk of missing the target.
Non-viral methods
Safer alternatives for gene delivery, such as lipid nanoparticles used in mRNA COVID-19 vaccines.
Electroporation
A method that allows gene editing without the use of viruses.
CAR-T cell therapy
Gene editing advancements that allow T-cells to be programmed to attack cancer.
Stem cells
A type of cell that can become any type of cell in a process called differentiation.
Embryonic stem cells
Pluripotent stem cells found in early-stage embryos that can turn into any cell type.
Adult stem cells
Multipotent stem cells that can differentiate into a limited range of cell types.
Adult Stem Cells
Stem cells found in tissues like bone marrow and blood that can only develop into specific cells relating to their origin.
Induced Pluripotent Stem Cells (iPSCs)
Stem cells created by reprogramming adult cells to act like embryonic stem cells, useful for personalised medicine.
Embryonic Stem Cells (ESCs)
Stem cells derived from early-stage embryos.
Adult Stem Cells (ASCs)
Stem cells found in tissues like bone marrow, skin, and blood.
Stem Cell Source Identification
The process researchers use to determine the type of stem cells they need.
Stem Cell Isolation and Culturing
The process of extracting and growing stem cells in controlled environments.
Extraction
The process of isolating cells from their source, such as embryos or bone marrow.
Culturing
The placement of cells in controlled environments with nutrients to grow and multiply.
Subculturing
The process of splitting cells into new culture dishes to continue growing when they reach confluence.
Characterisation and Differentiation
The process of identifying stem cells and inducing them to specialise into specific types.
Identification
The testing of stem cells to confirm their type and properties.
Differentiation
The process of inducing stem cells to specialise into specific types using chemical signals or genetic modifications.