H2 Chem (Theory)
1/1020
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
1021 Terms
Define proton number.
number of protons in the nucleus of each atom of an element
Define nucleon number / mass number.
total number of neutrons and protons in the nucleus of an atom of an element
State what is meant by an isotope.
Isotopes are atoms of an element that have the same proton number but different nucleon numbers.
Compare the properties of different isotopes.
Isotopes of an element have the same electronic configuration and chemical properties.
They have different relative isotopic masses and physical properties.
Which plate is an anion deflected to?
positive (+) plate
Which plate is a cation deflected to?
negative (-) plate
A cation has _____ electrons than protons.
fewer
An anion has _____ electrons than protons.
more
How to find the relative angle of deflection of a charged particle passing through an electric field?
𝜃 ∝ q/m
An electron, a neutron and a proton both enter a region with a uniform electric field.
State all the expected observations clearly.
The electron is deflected to the positive plate.
The proton is deflected to the negative plate.
The neutron passes straight through, experiencing no deflection.
The angle of deflection of the electron is much greater than the angle of deflection of the proton. This is because 𝜃 ∝ q/m, and the electron has the same magnitude of charge but a much smaller mass (1/1836 of proton).
Define relative atomic mass.
The relative atomic mass of an element is the ratio of the average mass of one atom of the element to 1/12 the mass of one atom of carbon-12.
Define relative isotopic mass.
The relative isotopic mass of an element is the ratio of the mass of one atom of the isotope to 1/12 the mass of one atom of carbon-12.
(There is no need to include the word ‘average’.)
How to find relative atomic mass (of an atom) using the relative isotopic mass of its isotopes?
Multiply each relative isotopic mass by its isotopic abundance. Add these numbers to obtain the relative atomic mass.
What is principal quantum number?
The principal quantum number describes the main energy level of an atom.
What is the formula for the maximum number of electrons that can occupy principal quantum shell n?
2n2
What is an atomic orbital?
An atomic orbital is a region of space with a 90% probability or more of finding an electron.
Each subshell (s, p, d, f) has one or more orbitals at the same __________ but different __________.
energy level (so they are degenerate); orientations in space
What is the shape of an s orbital?
spherical
What is the shape of a p orbital?
dumb-bell
How to draw d orbitals?
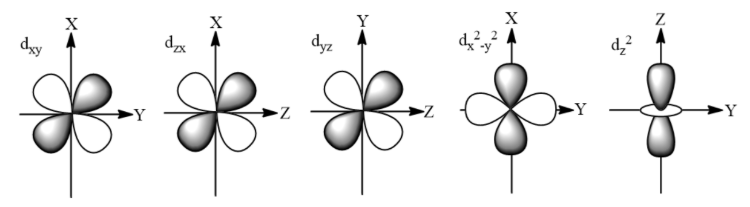
What is the order of subshells that electrons fill?
Start from 1s, end with 5s.
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, …
State the electronic configuration of Sc (proton number 21).
1s2 2s2 2p6 3s2 3p6 3d1 4s2
State the electronic configuration of V (proton number 23).
1s2 2s2 2p6 3s2 3p6 3d3 4s2
State the electronic configuration of Cr (proton number 24).
1s2 2s2 2p6 3s2 3p6 3d5 4s1
State the electronic configuration of Fe (proton number 26).
1s2 2s2 2p6 3s2 3p6 3d6 4s2
State the electronic configuration of Co (proton number 27).
1s2 2s2 2p6 3s2 3p6 3d7 4s2
State the electronic configuration of Cu (proton number 29).
1s2 2s2 2p6 3s2 3p6 3d10 4s1
State the electronic configuration of Zn (proton number 30).
1s2 2s2 2p6 3s2 3p6 3d10 4s2
Briefly explain why there are exceptions in the electronic configurations of Cr and Cu.
Increasing nuclear charge on 3d/4s orbitals, as well as repulsion between the two electrons in the 4s subshell.
Which subshell is at a lower energy level, 3d or 4s?
4s
Which subshell is closer to the nucleus, 3d or 4s?
3d
What is the method to deduce the electronic configuration of a cation?
First write the electronic configuration of the atom.
Next, remove the number of electrons equal to the positive charge, starting from the outermost shell (with the highest principal quantum number).
For transition elements, the 4s electrons are removed first.
What is the method to deduce the electronic configuration of an anion?
First write the electronic configuration of the atom.
Next, add the number of electrons equal to the negative charge, starting from the innermost shell.
To explain periodic trends, which factors should be considered in the explanation?
Number of principal quantum shells
Effective nuclear charge (equal to nuclear charge - shielding effect)
Explain the trend in atomic radii across a period.
Across a period, the effective nuclear charge increases due to the addition of protons, but the shielding effect remains relatively constant since the electrons are added to the outermost shell. Hence, effective nuclear charge increases and the electrostatic forces of attraction between the nucleus and the outermost electron become stronger.
Thus, the outermost electrons are pulled closer to the nucleus and atomic radii decreases across the period.
Explain the trend in atomic radii down a group.
Down a group, there is an addition of a principal quantum shell. The effective nuclear charge remains relatively constant since the increase in nuclear charge due to the addition of protons is offset by an increase in shielding effect as electrons added contribute to shielding. Hence, electrons are further away from the nucleus so the atomic radii increases down the group.
Explain the trend in atomic radii across the first row transition elements.
Across the first row transition elements, effective nuclear charge remains relatively constant because the increase in nuclear charge due to the addition of protons is offset by an increase in shielding effect since electrons are added to the inner 3d subshell and contribute to shielding. Hence, atomic radii remains relatively invariant across the first row transition elements.
Explain the trend in first ionisation energy across the period.
Across a period, the effective nuclear charge increases due to the addition of protons, but the shielding effect remains relatively constant since the electrons are added to the outermost shell. Hence, effective nuclear charge increases, so electrons are pulled more strongly to the nucleus and first ionisation energy increases.
However, there is a small dip between groups 2 and 13 because the p subshell is further away from the nucleus than the s subshell (so less energy is required to remove an electron from an element in group 13).
There is also a small dip between groups 15 and 16 because of interelectronic repulsion between the two electrons in the same p orbital (so less energy is required to remove an electron from an element in group 16).
Explain the trend in first ionisation energy down the group.
Down a group, there is an addition of a principal quantum shell. The effective nuclear charge remains relatively constant since the increase in nuclear charge due to the addition of protons is offset by an increase in shielding effect as electrons added contribute to shielding. Hence, electrons are further away from the nucleus, experience weaker attraction so first ionisation energy decreases down the group.
Explain the trend in first ionisation energy across the first row transition elements.
Across the first row transition elements, effective nuclear charge remains relatively constant because the increase in nuclear charge due to the addition of protons is offset by an increase in shielding effect since electrons are added to the inner 3d subshell and contribute to shielding. Hence, first ionisation energy remains relatively invariant across the first row transition elements.
Describe and explain the trend in ionic radii across the period.
Across the period, there are two sections of ionic radii: split into the cations and anions.
Among the cations, across the period, increasing nuclear charge and relatively constant shielding effect will increase effective nuclear charge, so ionic radius decreases.
Similarly, among the anions, across the period, increasing nuclear charge and relatively constant shielding effect will increase effective nuclear charge, so ionic radius decreases.
However, between the two sections, there is a huge jump in ionic radii due to the addition of a principal quantum shell, so the outermost electrons are further away from the nucleus.
Describe and explain the trend in ionic radii down the group.
Down a group, there is an addition of a principal quantum shell. The effective nuclear charge remains relatively constant since the increase in nuclear charge due to the addition of protons is offset by an increase in shielding effect as electrons added contribute to shielding. Hence, electrons are further away from the nucleus, experience weaker attraction so ionic radii increases down the group.
Define first ionisation energy.
The first ionisation energy is the energy required to remove one mole of electrons from one mole of gaseous atoms to form one mole of unipositively charged gaseous ions.
Define second ionisation energy.
The first ionisation energy is the energy required to remove one mole of electrons from one mole of unipositively charged gaseous ions to form one mole of gaseous ions with a double positive charge.
Explain why successive ionisation energies of a particular element always increase.
As electrons are removed from an element, nuclear charge remains the same while shielding effect decreases. Hence, effective nuclear charge increases, the electrons are pulled more strongly to the nucleus, and more energy is required to remove the next electron.
How to use successive ionisation energy values to determine the chemical property of an element?
Consider the big leaps in ionisation energies.
For example, if the first big leap is from the time the 2nd electron was removed to the time the 3rd electron was removed, this implies the 3rd electron is in an inner principal quantum shell. Hence, we can conclude that there are two electrons in the valence shell of the atom.
However, do take note: we are mainly concerned about big leaps. There may be some smaller but significant leaps (e.g. between the removal of the last 3p electron and the removal of 3s electron).
Define an ionic bond.
Ionic bonding is the electrostatic attraction between oppositely-charged ions (cations and anions).
Define a covalent bond.
Covalent bonding is the electrostatic attraction between the positively-charged nuclei of both bonding atoms and their shared electrons.
Define a metallic bond.
Metallic bonding is the electrostatic attraction between positively-charged metal cations and the sea of delocalised electrons.
Can you list all the types of electrostatic attractions?
metallic bonding
ionic bonding
covalent bonding
ion-dipole
pd-pd
pd-id
id-id (dispersion forces)
hydrogen bonding
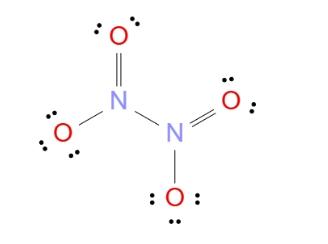
Draw the Lewis structure of N2O4, showing all lone pairs.
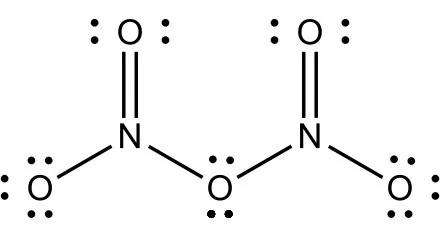
Draw the Lewis structure of N2O5, showing all lone pairs.
Define electronegativity of an atom.
Electronegativity of an atom is a measure of its ability to attract the electrons in a covalent bond to itself.
State and explain trends in electronegativity in the Periodic Table.
Across the period, electronegativity increases since effective nuclear charge is greater with a greater nuclear charge but a relatively constant shielding effect.
Down the group, electronegativity decreases due to the addition of principal quantum shells, causing electrons to be further away from the nucleus.
If there is a small difference in electronegativity between two atoms, what bond is likely formed?
covalent
If there is a large difference in electronegativity between two atoms, what bond is likely formed?
ionic
State the structure and bonding in metals.
Metals in the solid state have a giant metallic lattice structure which is a lattice of metal cations and a sea of delocalised electrons.
Strong electrostatic forces of attraction exist between metal cations and the sea of delocalised electrons.
What affects the strength of metallic bonding?
Number of valence electrons contributed per metal atom. The greater the number of valence electrons contributed per metal atom, the stronger the metallic bond.
The charge density (charge/radius) of the metal cation. The greater the charge density of the metal cation, the stronger the metallic bond.
State and explain whether Na or Mg has a higher melting point.
Mg.
Each atom of magnesium contributes 2 valence electrons in metallic bonding compared to only 1 valence electron contributed by each atom of sodium.
Mg2+ is of higher charge than Na+ and smaller radius (due to greater number of protons and effective nuclear charge), so the charge density of Mg2+ is greater than that of Na+.
Hence, more energy is required to overcome the stronger electrostatic forces of attraction between metal cations and the sea of delocalised electrons in Mg than Na.
Explain why metals have high melting and boiling points.
A large amount of energy is needed to overcome the strong electrostatic attraction between the metal cations and the delocalised electrons.
Are metals are good conductors of heat and electricity? Explain your reasoning.
The delocalised electrons can act as mobile charge carriers which may carry a current.
Metals are good conductors of heat because thermal energy causes the electrons to move more quickly, allowing for energy to be transferred to other parts of the metal.
State and explain the trend in the electrical conductivity across Period 3 elements from Na to Al.
Electrical conductivity increases.
Na, Mg and Al have giant metallic lattice structures.
From Na to Al, the number of valence electrons contributed per metal atom increases, hence electrical conductivity increases. (In addition, the charge density of the metal cation also increases.)
State what is meant by malleable.
able to be beaten or rolled into sheets
State what is meant by ductile.
able to be drawn in wires and tubes
Explain why metals are malleable and ductile.
When a large stress is applied to a metal, the layers of ions slide past one another into new positions.
The sea of delocalised electrons prevent repulsion between the cations, hence the metal will not break or shatter.
Explain why most metals used in practical scenarios are alloys.
Alloys are harder and stronger.
By incorporating small quantities of other elements into the pure metal, the orderly arrangement of a (pure) metal lattice is disrupted. Hence, when a force is applied, these ions can no longer slide over each other easily.
Describe the structure and bonding in ionic compounds.
Ionic compounds have a giant ionic lattice structure in the solid state, which is a lattice of cations and anions held together by ionic bonding.
Strong electrostatic forces of attraction exist between the cations and anions in ionic compounds.
The number of ions that surround another ion of the opposite charge in an ionic lattice is called the coordination number of that central ion.
Suggest two factors that affect coordination number in an ionic compound.
Relative sizes of ions. For instance, if an ion is large, then the other ion will have a smaller coordination number (as fewer ions can fit around it).
Relative charges of ions. Clearly, for a compound like CaF2, twice as many F is needed compared to Ca.
Among the elements in Group 14, the melting point of the elements show a decreasing trend down the group.
Account for this.
Increasing atomic size down the group due to the addition of principal quantum shells will lead to less effective atomic orbital overlap, hence the strength of covalent bonds decreases and there is a weaker attraction between the nuclei of bonding atoms and their shared electrons.
Define lattice energy.
Lattice energy is defined as the heat evolved when 1 mol of a pure solid ionic compound is formed from its constituent gaseous ions.
What affects the strength of ionic bonding?
The more exothermic the lattice energy (i.e. the greater the heat evolved), the stronger the ionic bond.
In turn, lattice energy is affected by the charges on the cations and anions, as well as the sum of the ionic radii of a cation and an anion.
Explain what affects lattice energy.
Lattice energy is proportional to the product of the charges and is inversely proportional to the sum of the ionic radii of the cation and anion.
Explain whether KCl or CaCl2 has a higher boiling point.
CaCl2
Ca2+ has a +2 charge, while K+ only has a +1 charge.
The ionic radius of Ca2+ is of a lower value compared to K+ as it has a higher nuclear charge (due to the presence of an additional proton).
Lattice energy is directly proportional to the product of ionic charges and inversely proportional to the sum of ionic radii.
Hence, more energy is required to overcome the stronger electrostatic forces of attraction in CaCl2 than in KCl.
Explain why ionic compounds have high melting and boiling points.
They have strong electrostatic forces of attraction which require a large amount of energy to overcome.
Are ionic compounds good conductors of electricity? Justify your answer.
Ionic compounds are good conductors of electricity in the aqueous and molten states since the cations and anions can act as mobile charge carriers.
However, in the solid state, these ions are not free to move since they are fixed in position in the giant ionic lattice structure, hence they cannot serve as mobile charge carriers.
Therefore, ionic compounds are not good conductors of electricity in the solid state.
The concentration of an aqueous ionic solution is increased.
State the effect, if any, on the electrical conductivity of this solution.
Electrical conductivity increases.
There are more ions that act as mobile charge carriers.
Explain why ionic compounds tend to be hard and rigid.
The ions are held in fixed positions throughout the giant ionic lattice. Hence, a large amount of force is required to move these ions out of their existing positions.
Explain why ionic compounds tend to be brittle.
When a sufficiently large force is applied, ions of like charges will be in close proximity with each other.
Their repulsion causes the lattice to shatter.
Explain what is meant by a covalent bond in terms of orbital overlap.
For a covalent bond to form, the orbitals of the bonding atoms must overlap.
Distinguish between a sigma bond and a pi bond.
A sigma bond is a head-on overlap, which is stronger and more effective compared to a pi bond which is a side-on overlap.
How to draw a sigma bond, showing the overlap?
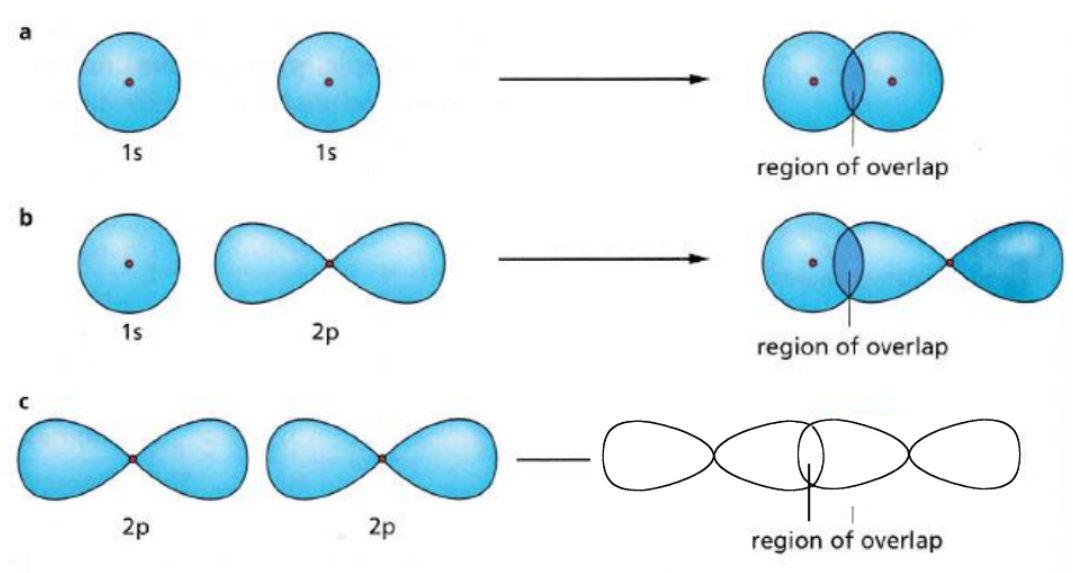
How to draw a pi bond, showing the overlap?
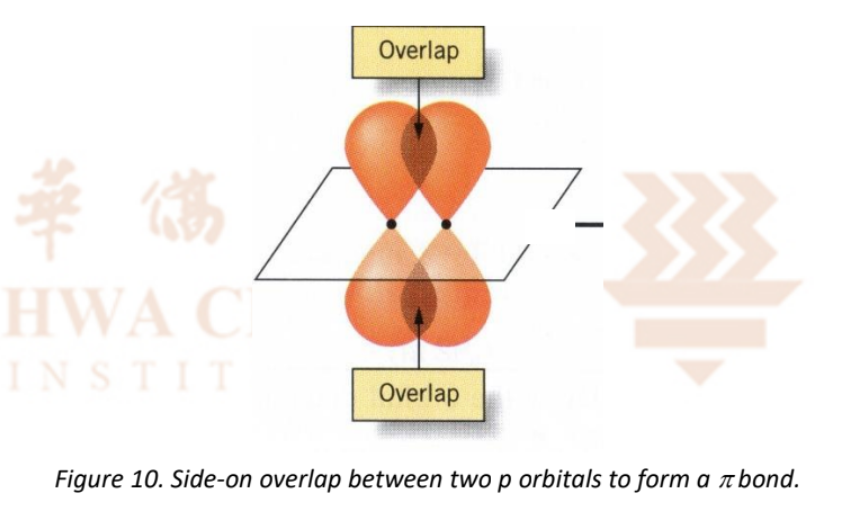
How many sigma and pi bonds are present in a double bond?
1 sigma, 1 pi
How many sigma and pi bonds are present in a triple bond?
1 sigma, 2 pi
How many sigma and pi bonds are present in propane?
10 sigma, zero pi
How many sigma and pi bonds are present in propene?
8 sigma, 1 pi
How is the strength of a covalent bond determined?
It is numerically determined by the bond energy.
Define bond energy.
Bond energy is the average amount of energy required to break 1 mole of a covalent bond in the gaseous state to form gaseous atoms.
How is bond energy affected by bond length and bond order?
Bond energy increases with a shorter bond length and a higher bond order.
With a greater bond order, more electrons are shared and the attraction is stronger.
With a shorter bond length, the overlap between atoms is more effective.
Explain why the radii of noble gases (e.g. as seen from a graph) is much larger than other elements in the same period.
The radii of noble gases given are van der Waals radii, which are between adjacent molecules.
However, the radii of other elements given are covalent radii which are found the same molecule.
Covalent radii are much shorter than the van der Waals radii.
Explain why sodium oxide has the formula Na2O.
Each Na atom loses one valence electron to form Na+.
Each O atom has six electrons in the valence shell, so it gains two electrons to form O2-.
The ratio of Na+ to O2- must be 2:1 for the resulting compound to be overall electrically neutral / to gain charge neutrality.
Hence, the empirical formula is Na2O.
Explain why some elements are able to hold more than 8 valence electrons (and expand their octet), while others are not.
Elements in Period 3 and above can form more than 4 bonds using energetically accessible d orbitals to accommodate additional electrons.
For elements in Period 2, d orbitals are not energetically accessible and cannot be used to accommodate additional electrons.
In SF4, how many electrons are there around the central atom?
10
Explain why chlorine can form ClF3 or ClF5 but not ClF4, ClF6 or ClF7.
It can form ClF3 or ClF5 by expanding its octet. Since chlorine is in Period 3, the d orbitals are energetically accessible. Hence, chlorine can accomodate more than 8 electrons.
However, ClF4 and ClF6 will result in an unpaired electron (radical), which is not feasible / the radical will readily react.
ClF7 cannot be formed since it is not large enough for 7 fluorine atoms to surround it.
To draw the dot-and-cross diagram, for each (+) charge, electrons are _______ from/to the _______ electronegative atom.
removed; less
To draw the dot-and-cross diagram, for each (-) charge, electrons are _______ from/to the _______ electronegative atom.
added; more
State the two main principles of the Valence Shell Electron Pair Repulsion (VSEPR) theory.
The electron groups around the central atom arrange themselves as far apart as possible to minimise electronic repulsion.
The repulsion between two lone pairs is greater than the repulsion between a lone pair and a bond pair, which is in turn greater than the repulsion between two bond pairs.
Why is a lone pair-lone pair repulsion more significant compared to a bond pair-bond pair repulsion? Explain in brief.
This is because lone pairs are only attracted by one nucleus, so they are less elongated compared to a bond pair which is attracted by both bonding nuclei.
Hence, a lone pair is closer to the nucleus it belongs to, and the repulsion is more significant.
State what is meant by bond angle.
Bond angle is the angle between two bond pairs in a molecule or ion.
State (1) the shape and (2) the bond angle(s) around a central atom with 2 bond pairs and no lone pairs.
linear, 180°