H Chemistry - Unit 1 - 1b - Chemical Changes and Structure
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
What is a covalent bond?
A covalent bond is the result of two positive nuclei being held together by their common attraction for the shared pair of electrons
When are polar covalent bonds formed?
Polar covalent bonds are formed when the attraction of the atoms for the pair of bonding electrons is different.
What are ionic bonds?
Ionic bonds are the electrostatic attraction between oppositely charged ions
What do ionic compounds form?
Ionic compounds form lattice structures of oppositely charged ions
What does the difference in electronegativities between bonded atoms give?
The difference in electronegativities between bonded atoms gives an indication of ionic character.
What type of bond are compounds formed between metals and non-metals?
Compounds formed between metals and non-metals are often, but not always, ionic.
What are Van der Waals forces?
Van der Waals forces are intermolecular forces acting between molecules
What are London Dispersion Forces?
London Dispersion Forces are forces of attraction that can operate between all atoms and molecules.
How are London Dispersion Forces formed?
London Dispersion Forces are formed as a result of electrostatic attraction between temporary dipoles and induced dipoles caused by the movement of electrons in atoms and molecules
What is the strength of London Dispersion Forces related to?
The strength of London Dispersion Forces related to the number of electrons within an atom or molecule
When is a molecule described as polar?
A molecule is described as polar when it has a permanent dipole
What can the spatial arrangement of polar covalent bonds result in?
The spatial arrangement of polar covalent bonds can result in a molecule being polar
What are permanent dipole-permanent dipole interactions?
Permanent dipole-permanent dipole interactions are additional electrostatic forces of attraction between polar molecules
What are hydrogen bonds?
Hydrogen bonds are electrostatic forces of attraction between molecules that consist of a hydrogen atom bonded to an atom of a strongly electronegative element such as fluorine, oxygen, or nitrogen. These are highly polar.
What three elements are present in hydrogen bonding?
The three elements present in Hydrogen Bonding are;
Fluorine
Oxygen
Nitrogen
Between polar and non-polar substances with a similar number of electrons, which type of substance has a higher melting point and boiling point?
The melting and boiling points of polar substances are higher than the melting and boiling points of non-polar substances with similar number of electrons
What are the boiling points, melting points, viscosity, and miscibility in water affected by?
Boiling points, melting points, viscosity, and miscibility in water are properties of substances that are affected by hydrogen bonding.
What are the anomalous boiling points of ammonia, water, and hydrogen fluoride a result of?
The anomalous boiling points of ammonia, water, and hydrogen fluoride are a result of hydrogen bonding
What does hydrogen bonding between molecules in ice result in?
Hydrogen bonding between molecules in ice results in an expanded structure that causes the density of ice to be less than that of water at low temperatures
Like dissolves like
Ionic compounds and polar molecular compounds tend to be soluble in polar solvents such as water.
Non-polar molecular subtances tend to be soluble in non-polar solvents and insoluble in polar solvents
What features can be used to predict the solubility of a compound
Features used to predict the solubility of a compound are;
Presence of O-H or N-H bonds in molecules, this implies hydrogen bonding
Spatial arrangement of polar covalent bonds, which could result in a molecule possessing a permanent dipole
Van der Waals forces in order of strength, from weakest to strongest
London Dispersion Forces
Permanent dipole-permanent dipole
Hydrogen bonding
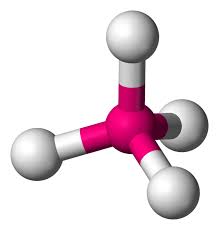
If the four outer atoms are the same element then will this molecule be polar or non-polar?
If the four outer atoms are the same element then that molecule will be non-polar
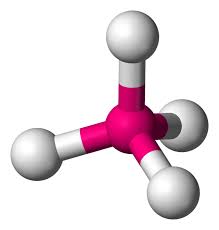
If the four outer atoms are not the same element then will this molecule be polar or non-polar?
If the four outer atoms are not the same element then that molecule will be polar
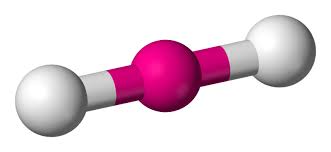
Will this molecule be polar or non-polar?
That molecule will be non-polar
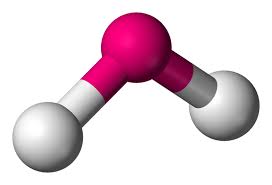
Will this molecule be polar or non-polar?
That molecule will be polar
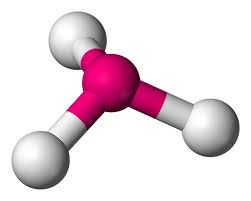
Will this molecule be polar or non-polar
That molecule will be polar
What does a symmetrical arrangement of dipoles result in?
A symmetrical arrangement of dipoles results in a non-polar molecule
What does a non-symmetrical arrangement of dipoles result in?
A non-symmetrical arrangement of dipoles results in a polar molecule