Glucose Metabolism and Citric Acid Cycle
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
67 Terms
Macronutrients
Needed in large amounts
Provide structural material and generate energy (for growth, movement and metabolic processes)
Carbohydrates, fats, proteins and water
Micronutrients
Needed in smaller quantities
Minerals and vitamins, cofactors for enzymes, antioxidants
How are different nutrients stored in the body?
Glucose is stored as glycogen in the liver and muscle
Fats are stored as triacylglycerols in adipose cells as a long-term energy reserve
Amino acids are used to make proteins and there is no special storage form. Excess amino acids are broken down to urea for excretion or oxidised for energy
Metabolism =
Catabolism (breakdown) + Anabolism (synthesis)
What is the major role of catabolism?
Oxidise food to provide energy
What is the major role of anabolism
Convert food molecules into new cellular material
5 steps of glucose metabolism
Glycolysis
Pyruvate
Gluconeogenesis
Pentose phosphate pathway
Glycogen
What is the main macromolecule in metabolism?
Glucose
Why and how is glucose used to produce energy?
Glucose is rich in potential energy (how much energy it could produce)
It is stored as a high molecular weight polymer such as starch or glycogen
When energy demands increase, glucose is released and used to produce energy
Glycolysis
What happens in glycolysis overall?
A molecule of glucose (6C) is broken down in a series of enzyme-catalysed reactions
Produces 2 molecules of pyruvate (3C)
Where does glycolysis occur?
In the cytosol (cytoplasm of cells)
Glycolysis
2 phases
10 steps divided into 2 phases:
Preparatory phase
Payoff phase
Glycolysis
Preparatory phase
Glucose is phosphorylated at the hydroxyl group on C6 (using a molecule of ATP hydrolysed to ADP and Pi), forming glucose-6-phosphate
Glucose 6-phosphate is converted to fructose 6-phosphate
Fructose 6-phosphate is phosphorylated at C1 (using a molecule of ATP) forming fructose 1,6-biphosphate
Fructose 1,6-biphosphate is split to produce 2 different 3C molecules » dihydroxyacetone phosphate and glyceraldehyde 3-phosphate (this is the lysis step)
The dihydroxyacetone phosphate is isomerised to form a second molecule of glyceraldehyde 3-phosphate
Products:
2 molecules of glyceraldehyde 3-phosphate
2 ATP used up
Glycolysis
Payoff phase
Each molecule of glyceraldehyde 3-phosphate is oxidised (loses e-) and phosphorylated by inorganic phosphate (NOT from ATP) to form 1,3-biphosphoglycerate. 2 NADs accept these e- and are converted to NADH
The 2 molecules of 1,3-biphosphoglycerate are converted to 2 molecules of pyruvate, releasing energy (4 ADPs are converted to ATP)
Products
2 molecules of pyruvate
4ATP - 2ATP used in preparatory phase = net yield of 2 ATP
2 NADH
Glycolysis
Overall reaction
Glucose + 2NAD+ + 2ADP + 2Pi —> 2 pyruvate + 2NADH + 2H+ +2ATP + 2H2O
Glycolysis
Why does glycolysis release only a small fraction of the total available energy of the glucose molecule?
The 2 molecules of pyruvate formed still contain most of the chemical potential energy of glucose
Glycolysis
How do each of these carbohydrates form glucose that can then enter glycolysis
Dextrin
Maltose
Lactose
Sucrose
Trehalose
Carbohydrates have to undergo hydrolysis to form monosaccharides
This is because only monosaccharides are taken up from the intestine
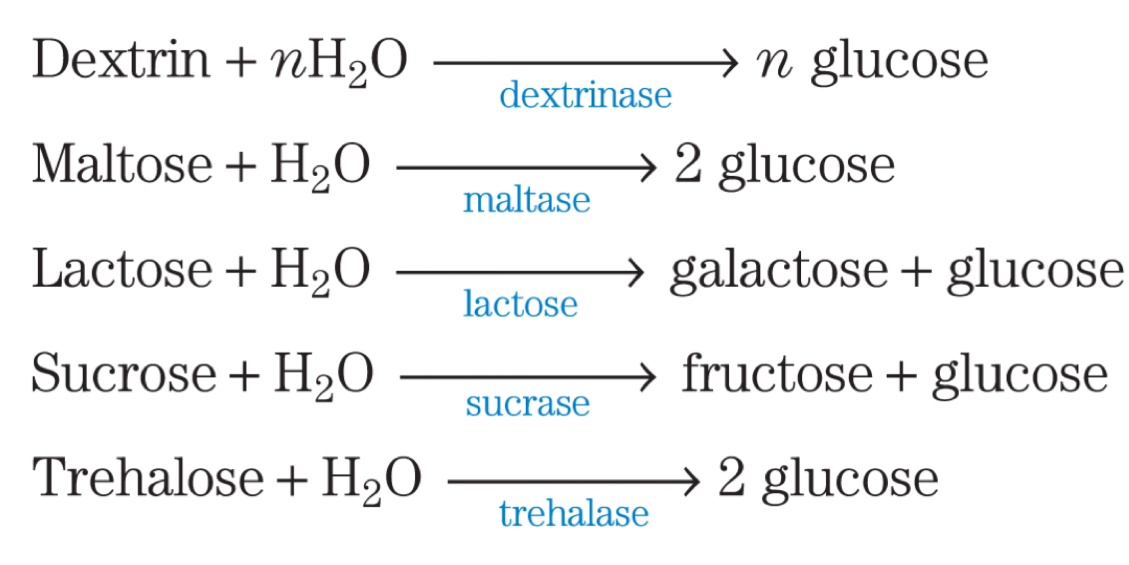
Lactose intolerance
Lactase converts lactose into glucose & galactose for absorption.
Most adults lose lactase after childhood, except in certain populations (e.g., Northern Europe, parts of Africa).
Without lactase, lactose cannot be completely digested and absorbed into the small intestine and it passes into the large intestine, where bacteria convert it to toxic products that cause abdominal cramps and diarrhoea
Diagnosis:
Blood glucose test — if lactase is present, lactose ingestion should increase glucose = not lactose intolerant
What 3 catabolic routes is pyruvate further metabolised via after glycolysis?
Under aerobic conditions, pyruvate is then oxidised to acetyl-CoA which then enters the citric acid cycle
Under anaerobic conditions:
Pyruvate is reduced to lactate (lactic acid fermentation)
Pyruvate is catabolised to ethanol and CO2 (ethanol fermentation)
» in both NAD+ is regenerated
Lactic fermentation
Equation
Glucose —> 2Lactic acid + 2ATP
Lactic fermentation
Describe the process
Glucose is converted to pyruvate
Pyruvate is reduced to form lactate, so NADH is oxidised to NAD+ (pyruvate accepts H lost by NADH)
NAD+ used in glycolysis
Lactate loses a H+ to form lactic acid
Ethanol fermentation
Equation
Glucose —> 2 ethanol + 2 carbon dioxide + 2 ATP
C6H12O6 —> 2C2H5OH + 2CO2
Ethanol fermentation
Describe the process
Glucose is converted to pyruvate
Pyruvate is decarboxylated, forming ethanal and carbon dioxide
Ethanal is reduced to form ethanol, so NADH is oxidised to NAD+ (pyruvate accepts H lost from rNAD)
NAD+ used in glycolysis
If an organism was to switch from aerobic respiration to alcoholic fermentation, what would happen to the amount of CO2 produced in a certain amount of time?
Amount of CO2 produced would increase
Aerobic respiration is more efficient than anaerobic
So more ATP is produced per molecule of glucose
So for anaerobic to produce enough ATP it has to respire more, increasing production of CO2
Why is pyruvate decarboxylase present in brewer’s and baker’s yeast?
Yeast ferments glucose to ethanol and CO2 in ethanol fermentation
CO2 is responsible for the carbonation of champaign
CO2 causes dough to rise in baking
What is alcohol dehydrogenase used for?
Present in many organisms that metabolise ethanol
In the liver, it catalyses the oxidation of ethanol
This reduces NAD+ to NADH
What happens when supply of glucose is not sufficient to produce enough energy for the body?
Glucose is synthesised from non-carbohydrate precursors e.g. lactate, pyruvate and glycerol in gluconeogenesis
Gluconeogenesis
Lactate, pyruvate and glycerol are all…
3C compounds
Gluconeogenesis
Where does gluconeogenesis occur?
Mainly in the liver
Renal cortex
In the epithelial cells that line the small intestine
Gluconeogenesis
Gluconeogenesis of lactate
Lactate is produced by anaerobic glycolysis in the skeletal muscles after vigorous exercise
It returns to the liver and is converted to glucose which then goes to the muscles
Gluconeogenesis
7 of the 10 enzymatic reactions of gluconeogenesis are the reverse of glycolysis.
Which 3 reactions of glycolysis are irreversible in vivo and cannot be used in glycolysis?
Conversion of glucose to glucose 6-phosphate
Phosphorylation of fructose 6-phosphate to fructose 1,6-biphosphate
Conversion of phosphoenolpyruvate to pyruvate
Gluconeogenesis
Why do both glycolysis and gluconeogenesis not occur at the same time in the same tissue?
If both reactions happened at the same time, a large amount of ATP would be consumed and energy lost as heat
Hence, glycolysis and gluconeogenesis are reciprocally regulated
What is the pentose phosphate pathway?
When the body does not need ATP, glucose 6-phosphate can enter an alternative metabolic pathway to form:
5C sugar ribose 5-phosphate » used to make RNA, DNA, ATP etc
NADPH » used to make fatty acids, cholesterol and steroid hormone
Takes place in the cytosol
Glycogen
The storage form of glucose in animals
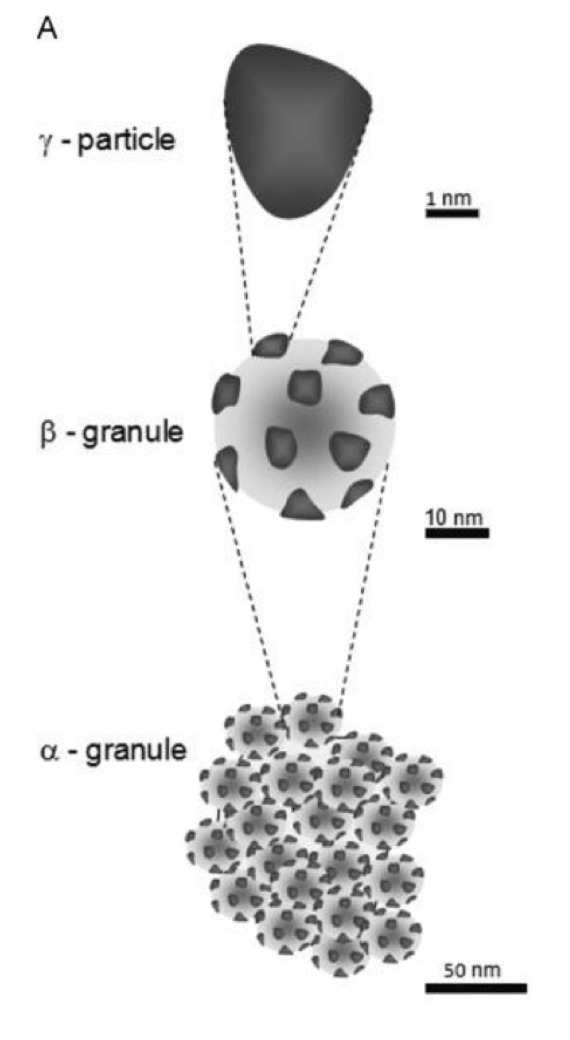
How is glycogen stored?
Stored as cytosolic granules called β-granules.
In the liver, 20 to 40 β-granules cluster to form α-granules.
γ-particles are associated with each β-granule, which contain enzymes for glycogen synthesis & breakdown
What enzymes are involved in glycogen breakdown to glucose?
Glycogen phosphorylase → Converts glycogen to G1P.
Glycogen debranching enzyme → Removes branches.
Phosphoglucomutase → Converts G1P to G6P.
What happens to glycogen in muscle vs. liver?
Skeletal Muscle: G6P enters glycolysis to release energy for muscle contraction
Liver: Glycogen breakdown releases glucose into blood when levels drop.
Where does glycogen synthesis occur?
In all animal tissues
But predominantly in the liver and skeletal muscles
Describe the process of glycogen synthesis
Glycogenesis occurs primarily in the liver and skeletal muscles.
Glucose is converted to glucose-6-phosphate (G6P).
G6P is converted to glucose-1-phosphate (G1P).
G1P is activated to form UDP-glucose.
Glycogenin acts as a primer.
Glycogen synthase adds glucose units to the glycogen chain.
A branching enzyme introduces α-1,6 linkages, creating a branched glycogen structure.
Regulation of breakdown of glycogen to glucose
How is the breakdown of glycogen to glucose regulated?
Glycogen phosphorylase is the enzyme that breaks down glycogen into glucose
2 forms:
glycogen phosphorylase a which is active
glycogen phosphorylase b which is less active
Its activity is controlled by phosphorylation, which is influenced by hormones like glucagon (in the liver) and epinephrine
Regulation of breakdown of glycogen to glucose
How do both epinephrine and glucagon control this?
Epinephrine:
After vigorous muscle activity, epinephrine triggers the phosphorylation of phosphorylase b converting it to a
This stimulates glycogen breakdown and glycolysis
Provides ATP for muscle contraction
Glucagon:
In the liver, glucagon phosphorylates phosphorylase b converting it to a
This stimulates glycogen breakdown to form glucose
This also stimulates gluconeogenesis (formation of glucose from other sources)
However, glucagon inhibits glycolysis in the liver, ensuring that glucose stays in the bloodstream for other parts of the body (especially the brain)
Why is glycogen a necessary source of energy in vertebrate animals?
Even though animals store 100 times more energy as fat than as glycogen, they cannot convert fats to glucose
A sudden burst of physical activity demands a quick source of energy in the muscles » glycogen quickly converted to glucose for glycolysis
Between meals or during a fast » glycogen releases glucose to provide a steady supply of glucose in the blood
Important for the brain » cannot use fatty acids for energy as long chain fatty acids do not cross BBB
In aerobic conditions, what is the next step after glycolysis?
Pyruvate is converted to Acetyl-CoA in the link reaction
Link Reaction
What is the overall reaction known as?
Oxidative decarboxylation
» An irreversible oxidation process where the carboxyl group is removed from pyruvate as a molecule of CO2
» And the 2 remaining Cs become the acetyl group of acetyl-CoA
Link Reaction
Overall reaction
Pyruvate (3C) —> CO2 + Acetyl (2C)
Acetyl + Coenzyme A —> Acetyl CoA
Link Reaction
Describe how pyruvate is converted to Acetyl-CoA
Pyruvate is oxidised to Acetyl-CoA and CO2 by the pyruvate dehydrogenase (PDH) complex » complex of E1, E2 and E3 enzymes.
Pyruvate remains bound to the PDH complex throughout the process
Pyruvate moves via a carrier protein into the mitochondrial matrix from the cytosol by active transport
The E1 enzyme catalyses the decarboxylation of pyruvate, removing 1C to form CO2 and forming a 2C acetyl group
The acetyl group is oxidised
The E2 enzyme binds the C2 acetyl group to coenzyme A, forming Acetyl-CoA
The oxidation of the acetyl group releases 2 high-energy e⁻, which are retained by the E2 enzyme. These electrons are then passed to NAD⁺, reducing it to NADH.This step is catalysed by E3 enzyme in the PDH complex.
NADH travels through the mitochondrial matrix, delivering electrons to the electron transport chain (ETC) for ATP production.
Acetyl-CoA proceeds into the Krebs cycle
Link Reaction
Overall products
Each pyruvate undergoes a separate link reaction so:
2 CO2
2 NADH
2 Acetyl-CoA
Link Reaction
How many reactions take place?
5 consecutive reactions
Link Reaction
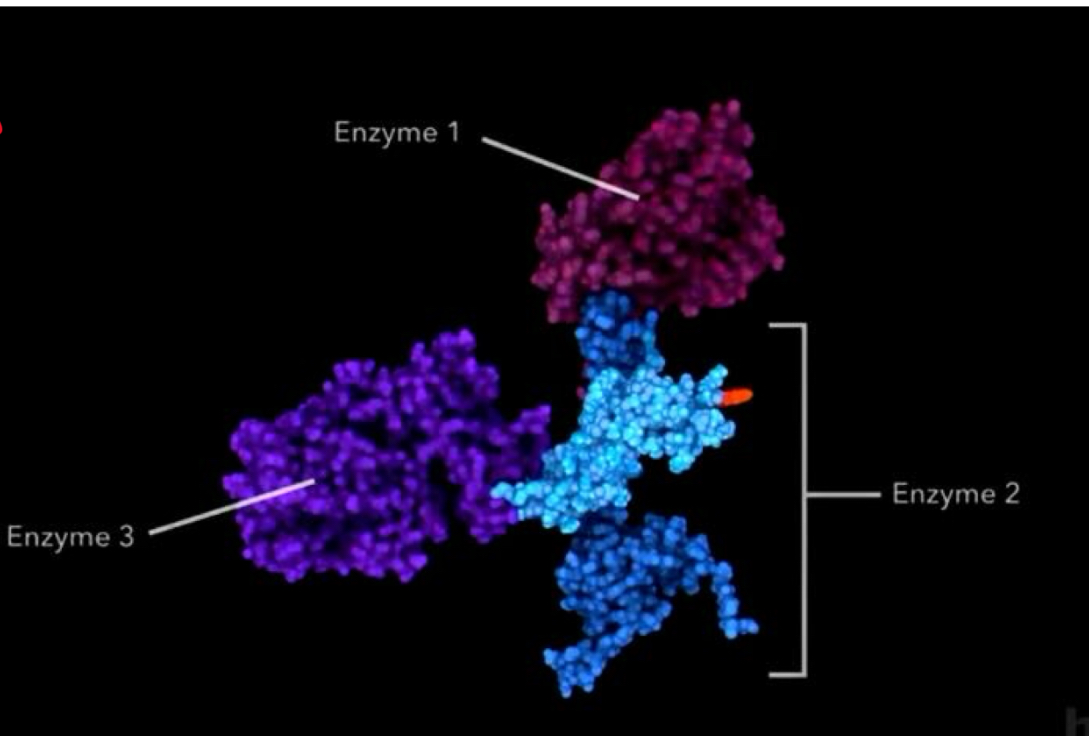
Label the E1, E2 and E3 enzymes on the PDH complex
Link Reaction
Name the different enzymes involved in the PDH complex
E1 - pyruvate dehydrogenase
E2 - dihydrolipoyl transacetylase
E3 - dihydrolipoyl dehydrogenase
Link Reaction
State the 5 different coenzymes involved in the link reaction
TPP (contains the vitamin thiamine)
CoA (contains the vitamin pantothenate)
FAD (contains the vitamin riboflavin)
NAD (contains the vitamin niacin)
Lipoate
Which four vitamins are essential for the pyruvate dehydrogenase complex, and what happens if one is deficient?
The four vitamins essential for the PDH complex are:
Thiamine (TPP)
Pantothenate (CoA)
Riboflavin (FAD)
Niacin (NAD)
A deficiency in thiamine (Vitamin B1) leads to impaired pyruvate oxidation
This is particularly harmful to the brain as it relies on glucose oxidation for energy.
Beriberi, a disease caused by thiamine deficiency, results in muscle weakness, nerve damage, and heart failure.
Wernicke’s syndrome (linked to alcoholism) can cause confusion, coma, and death.
High pyruvate levels in the blood may indicate a defect in pyruvate oxidation
Which step occurs after the Link Reactiom?
The Citric Acid Cycle / Krebs Cycle
Citric Acid Cycle
How many steps are in the cycle?
8 steps
Citric Acid Cycle
Describe the citric acid cycle
For every glucose, the citric acid cycle occurs TWICE
Acetyl-CoA (2C) donates its acetyl group to oxaloacetate (4C), forming citrate (6C).
Citrate is rearranged into isocitrate (6C) to prepare for oxidation.
Isocitrate undergoes oxidatiion & decarboxylation, losing CO₂ and forming α-ketoglutarate (5C). NAD⁺ is reduced to NADH in the process.
4. α-Ketoglutarate (5C) loses another CO₂, forming succinate (4C). NAD⁺ is reduced to NADH and Coenzyme A is added temporarily, forming Succinyl-CoA.
Succinyl-CoA is converted to succinate, forming ATP
Succinate is oxidised to fumarate (4C), reducing FAD to FADH₂
Fumarate is hydrated to malate (4C)
Malate is oxidised to regenerate oxaloacetate (4C), coupled with the reduction of NAD⁺ to NADH.
The cycle is now ready to restart with another Acetyl-CoA.
Citric Acid Cycle
Diagram
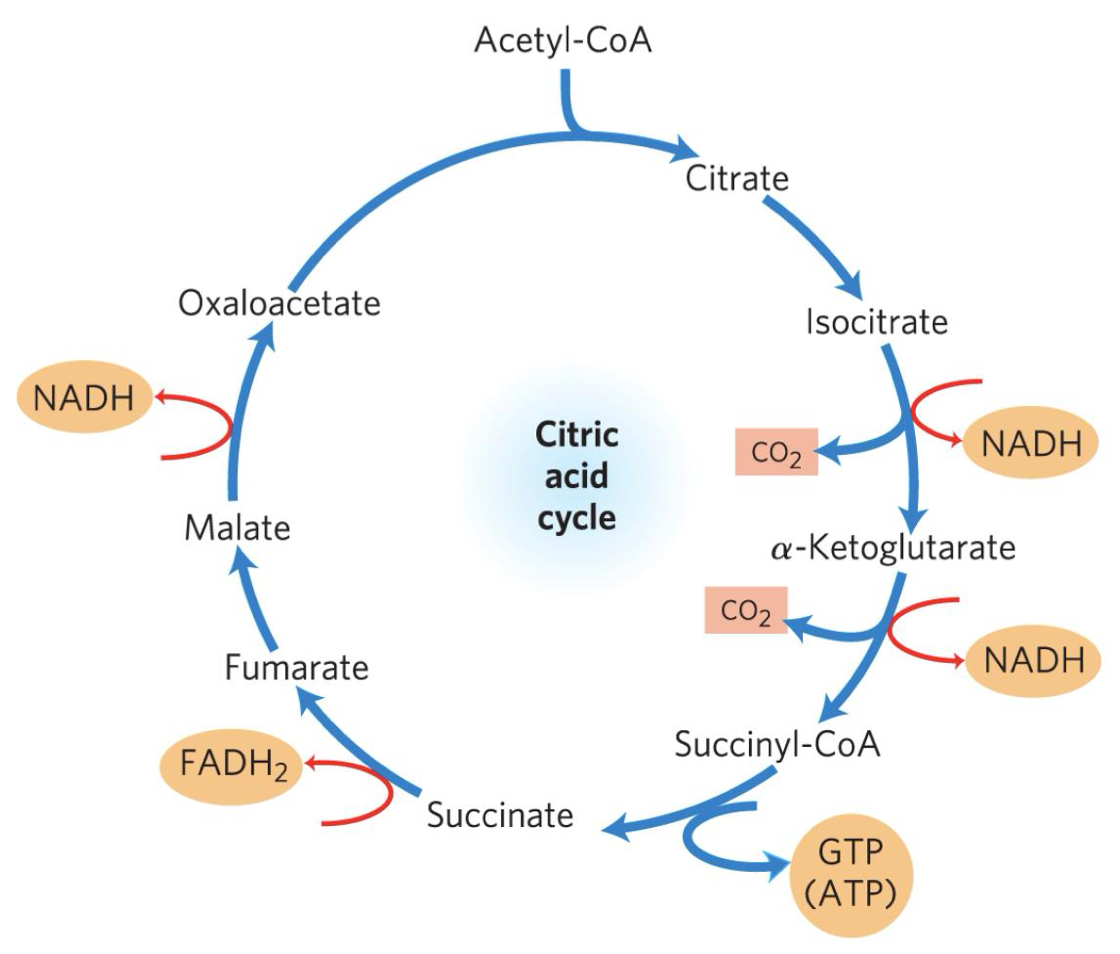
Citric Acid Cycle
Overall products
Each cycle produces:
3 NADH
1 FADH2
1 ATP
2 CO2
So per glucose (for 2 Acetyl-CoAs):
6 NADH
2 FADH2
2 ATP
4 CO2
Citric Acid Cycle
What is the energy gain for each Acetyl-CoA oxidised by the citric acid cycle?
3 NADH
1 FADH2
1 ATP
Citric Acid Cycle
How many of the 8 steps are oxidation reactions?
How is energy conserved?
4 out of the 8 steps are oxidation reactions
In which the energy is efficiently conserved in the form of the reduced coenzymes: NADH and FADH2 (accept the e- lost)
Citric Acid Cycle
Purpose of NADH and FADH2
Donate their e- to the ETC to form ATP
Citric Acid Cycle
Why is the citric acid cycle an amphibolic pathway?
It is both catabolic and anabolic
Anabolic:
Oxaloacetate and α-ketoglutarate can be withdrawn from the cycle to act as precursors for amino acids such as aspartate and glutamate
Succinyl-CoA is an intermediate in the synthesis of the porphyrin ring of heme groups
Ocxaloacetate can be converted to glucose via gluconeogenesis
Citric Acid Cycle
What are anaplerotic reactions?
Metabolic reactions that replenish intermediates of the Citric Acid Cycle
Withdrawal of intermediates for use in biosynthesis lowers the concentration of them enough to slow the cycle
So the intermediates can be replenished by anaplerotic reactions (‘to refill’)
E.g. Glutamate —> α-ketoglutarate
E.g. Propionyl-CoA → Succinyl-CoA
Citric Acid Cycle
How is the citric acid cycle regulated?
The production of Acetyl-CoA by the PDH complex can be:
Inhibited allosterically by metabolites that signal enough energy has been produced
Stimulated allosterically by metabolites that signal there is reduced energy supply
Citric acid cycle in tumours
Tumour cells block pyruvate from entering mitochondria, so it builds up in the cytosol.
Instead of going through the citric acid cycle, pyruvate is turned into lactate (which stimulates tumour growth).
PDH and succinate dehydrogenase are inactivated, causing a buildup of lactate & succinate (oncometabolites that promote tumour growth)
Mutations in citric acid cycle enzymes can lead to tumours in the adrenal gland, kidney, and muscle.
Each NADH produces how much ATP?
2.5 ATP
Each FADH2 produces how much ATP?
1.5 ATP
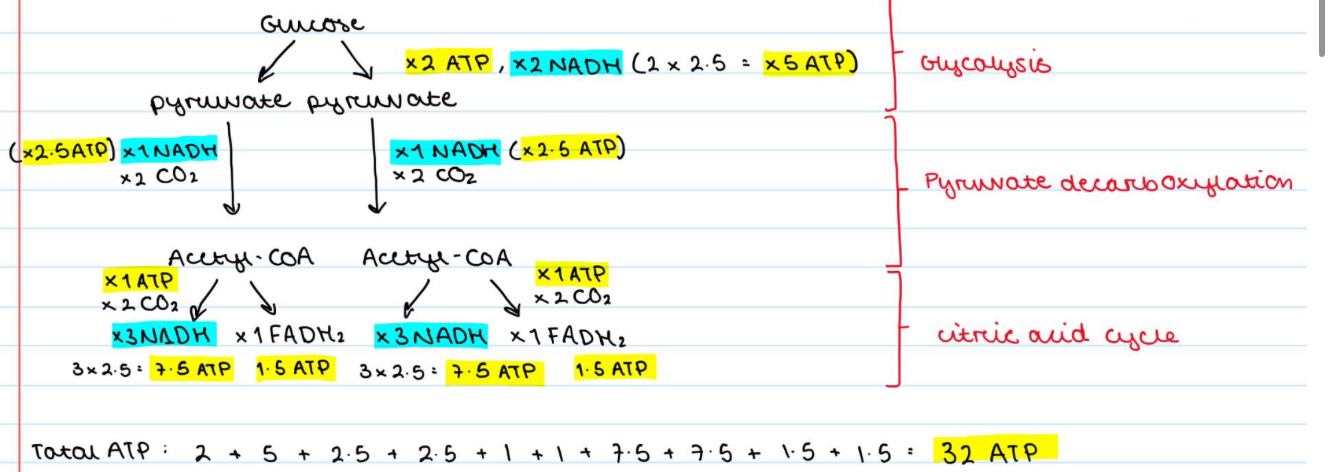
How much ATP is produced by the uptake and full oxidation of 1 molecule of glucose via glycolysis, PDH and the TCA cycle in a cell?