Chemistry - Chapter 2 Measurements and Calculations
0.0(0)
Card Sorting
1/63
There's no tags or description
Looks like no tags are added yet.
Last updated 2:57 PM on 8/25/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
1
New cards
Model
Explanation of how phenomena occur. Not real.
2
New cards
Theory
Broad generalization that explains a body of facts or phenomena
3
New cards
Hypothesis
educated guess of the cause of the problem or answer to the question.
4
New cards
Experiment
designed to test the hypothesis
5
New cards
Which stages of scientific method involve data?
all
6
New cards
What might be wrong if repeated tests don’t support hypothesis?
hypothesis might be wrong
7
New cards
Two parts of a metric system:
A prefix and a base unit
8
New cards
what does a prefix tell you?
how many times to divide or multiply by 10.
9
New cards
In SI measurment, dont use what?
Don’t use commas because this means a \n decimal point in some countries. Use a space instead. 75,000 is written as 75 000. (with a space)
10
New cards
deci d =
1/10 (10 to the -1)
11
New cards
centi c =
1/100 (10 to the -2)
12
New cards
milli m
1/1000 (10 to the -3)
13
New cards
micro μ
10 to the -6
14
New cards
a derived unit
the properties of base unit ( cm squared, square unit)
15
New cards
In a beaker, there are 8 different substances which substance is denser?
the one on the bottom (its heavier)
16
New cards
What state of matter is mercury?
liquid
17
New cards
What state of matter is ice?
solid
18
New cards
D = mass/volume can be simplified to
g/ml or g/cm 3 (cubed) Note: 1 mL = 1 cm 3 (cubed)
19
New cards
What is the density of osmium if 50g of the metal occupies a volume of 2.22cm 3 cubed?
22\.5 g/cm3
20
New cards
8 cm 3 (cubed) =
8 mL
21
New cards
48g/8mL to density (g/cm3)?
6 g/cm3
22
New cards
Water freezes at what temp?
0 C
23
New cards
Celsius formula
C = K - 273
24
New cards
Kelvin formula
K = C + 273
25
New cards
Dimensional Analysis
a **method of analysis in which physical quantities are expressed in terms of their fundamental dimensions of length, mass, and time**
26
New cards
How many m 3 (subed is 1500 cm 3 (cubed)
0\.0015m 3 (cubed)
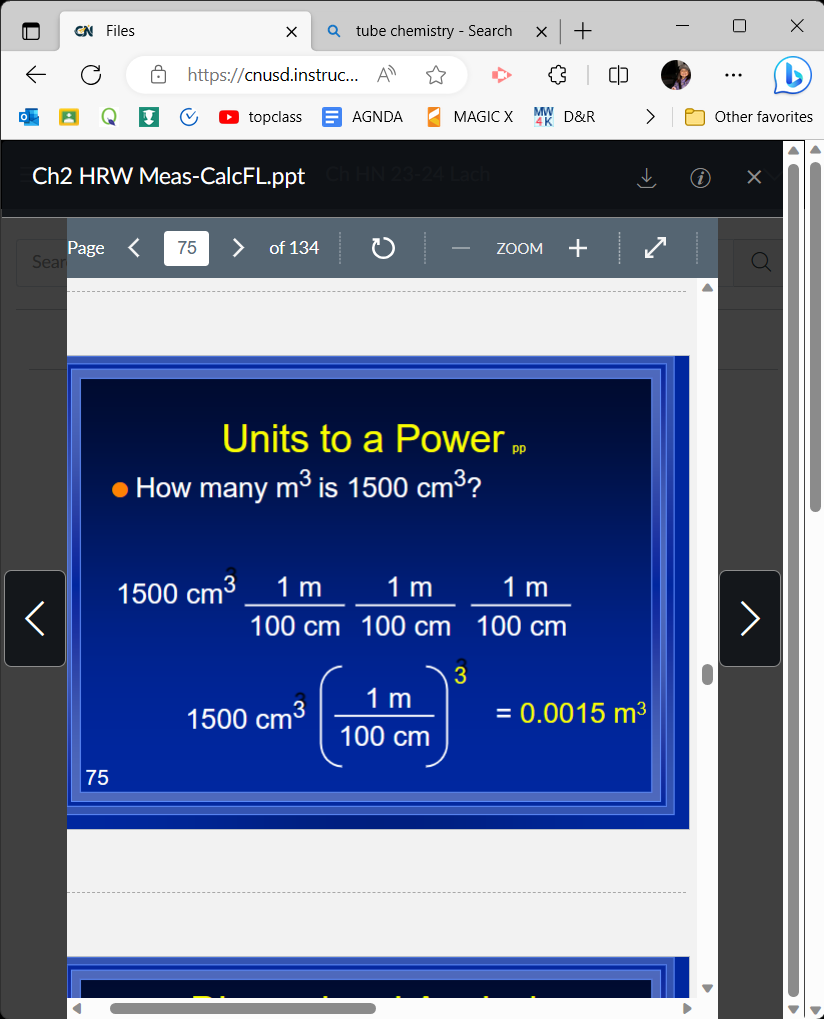
27
New cards
6 ft =
1 fathom
28
New cards
Accuracy
how close the measurement is to the actual value
29
New cards
Precision
how well can the measurement be repeated
30
New cards
How many sig figs in 12400?
3
31
New cards
How many sig figs in 0.045?
2
32
New cards
How many sig figs in 3440. cm?
4
33
New cards
How many sig figs in 0.006 700 0 kg?
5
34
New cards
Do measurements have sig figs?
Yes, Only measurements have sig figs
35
New cards
Counted numbers
exact (infinite significance)
ex: a dozen donuts, a paper measured 11 inches
ex: a dozen donuts, a paper measured 11 inches
36
New cards
Is gold melting at 1064°C exact or measured?
measured
37
New cards
Is 1 yard = 3 ft exact or measured?
exact
38
New cards
How many sig figs in 250 pencils
Infinite because its counted
39
New cards
Round 45.462 to four sig figs
45\.46
40
New cards
Round 45.462 to three sig figs
45\.5
41
New cards
Round 45.462 to two sig figs
45
42
New cards
Round 45.462 to one sig fig
50
43
New cards
If rounding from a “final 5”, then round to an
even number
44
New cards
Round 78.65 to 3 sig figs
Rounds down to 78.6 (even number)
45
New cards
Round 78.75 to 3 sig figs
Rounds up to 78.8 (because its an even number)
46
New cards
Round 4.652 to 2 sig figs
4\.7 (its not a final 5)
47
New cards
When add/subtracting sig figs,
The # with the lowest decimals value determines the place of the last sig fig in the answer
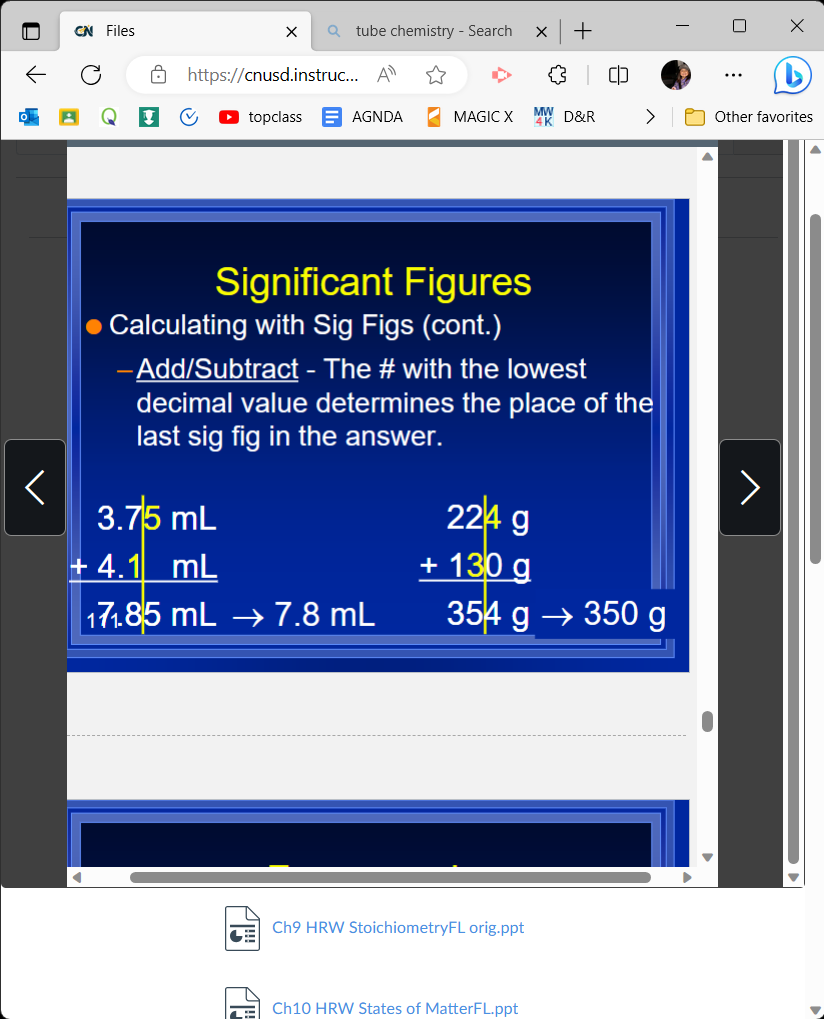
48
New cards
27\.93 + 6.4 (sig figs)
34\.3

49
New cards
When mult/diving sig figs,
the # with the lowest sig figs determines the answers amount of sig figs
50
New cards
3\.6 x 653
2400 (rounded
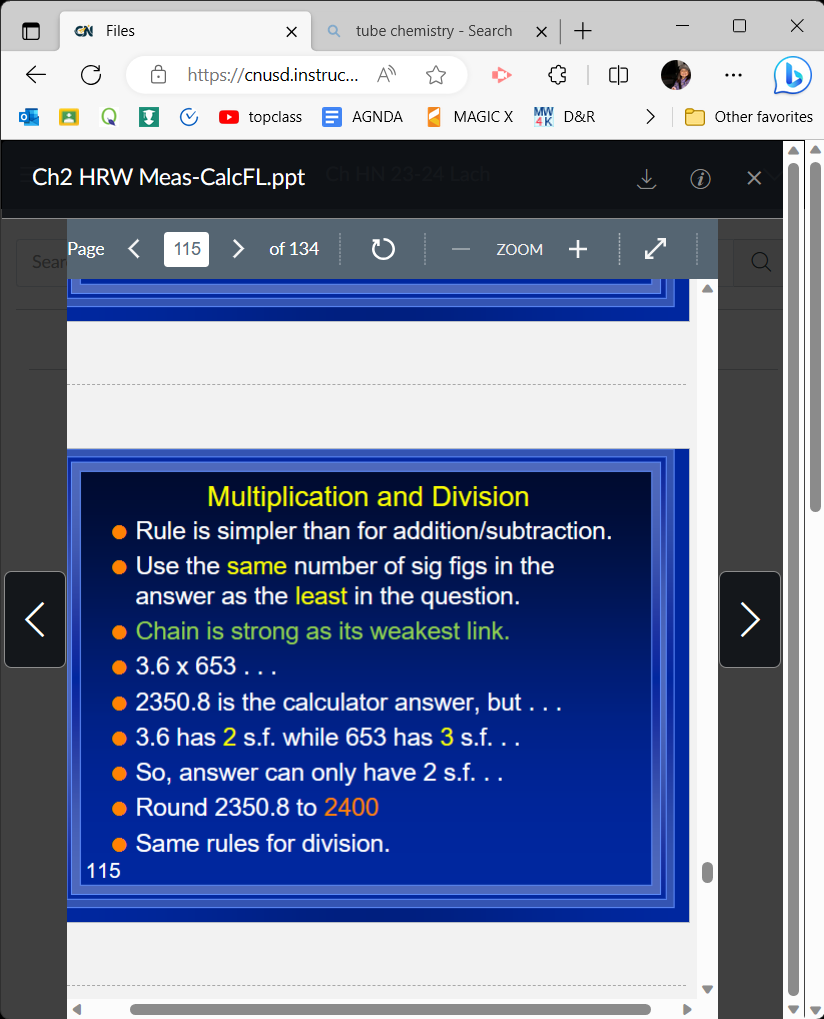
51
New cards
4\.5/6.245
0\.72
52
New cards
9\.8764 x .043
0\.42
53
New cards
Problem solving step 1
\
Analyze: Identify the unknown
\
Analyze: Identify the unknown
\
54
New cards
Problem solving step 2
Plan a solution
The “heart” of problem solving
Break it down into steps.
Look up needed information
The “heart” of problem solving
Break it down into steps.
Look up needed information
55
New cards
Problem solving step 3
Compute (calculate) - cancel units, sig figs
56
New cards
Problem solving step 4
Evaluate-
Sig Figs correct,
Units correct,
Check your work,
Reread the question, did you answer it,
Is it reasonable? Estimate (check order of magnitude)
Sig Figs correct,
Units correct,
Check your work,
Reread the question, did you answer it,
Is it reasonable? Estimate (check order of magnitude)
57
New cards
Direct proportions formula
y/x=k or y=kx
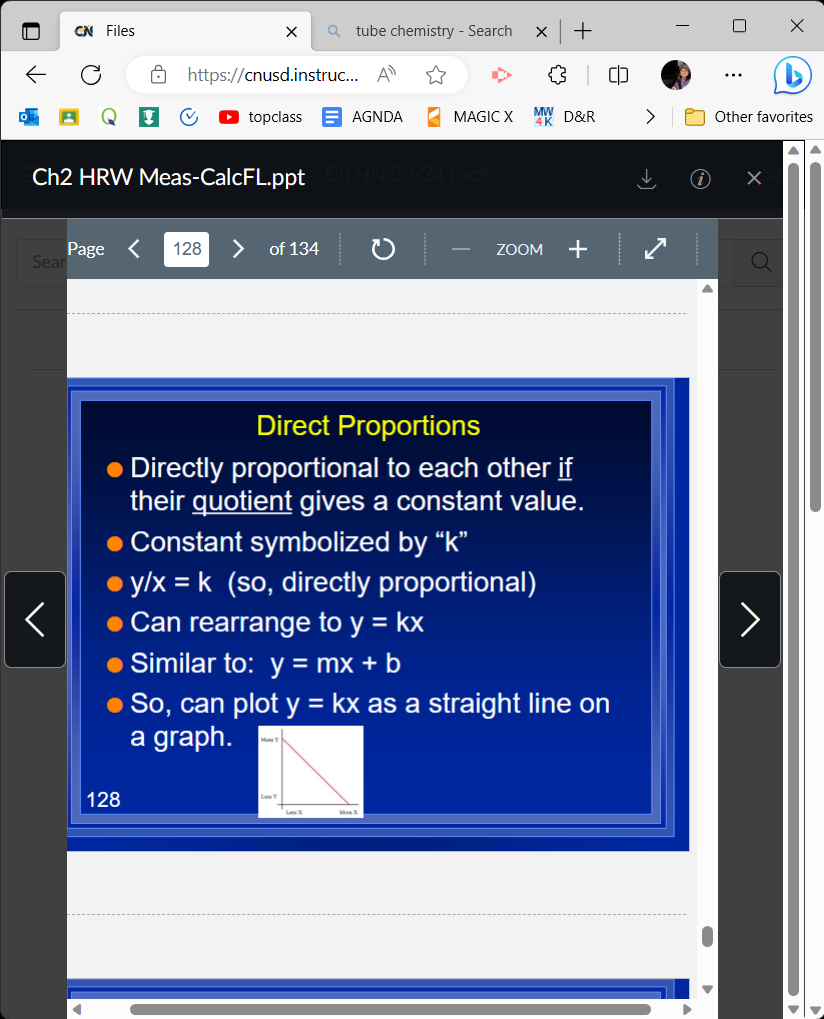
58
New cards
Indirect proportions formula
xy=k
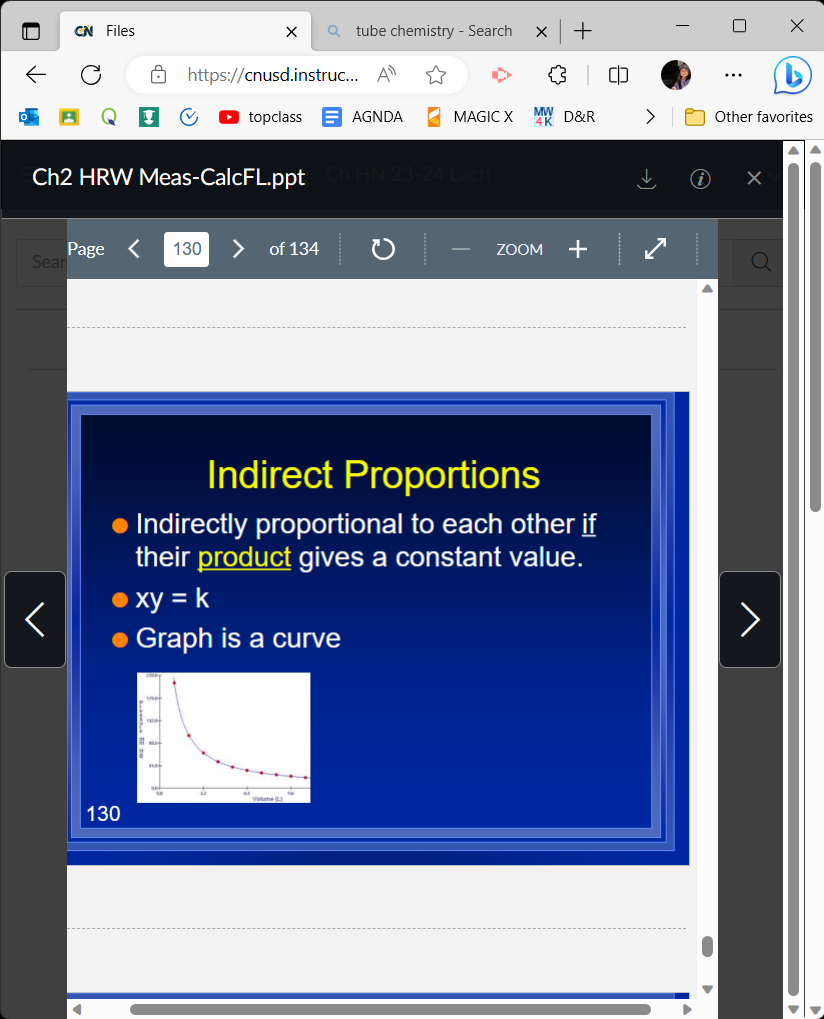
59
New cards
In sig figs if there is a 5, round up only if
the number before the 5 is odd
60
New cards
In sig figs if there is a 5, let the number be (dont round up) only if
the number before the 5 is even
61
New cards
Convert 31.95 x 10^3kg to Scientific Notation
3\.195 x 10^4
62
New cards
Convert 500,000 cm 3 (cubed) → m3 (cubed)
0\.5 cm 3
63
New cards
Convert 2.4 x 10 to the 8th cm 3 (cubed) → m 3 (cubed)
2\.4 x 10 to the 2nd m 3 (cubed)
64
New cards
Convert 8.92m 3 (cubed) in cm 3 (cubed)
8,920,000 cm 3 (cubed)