EXP 5: Distillation
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
Distillation
Process used to recover a liquid from a dissolved non-volatile impurity by heating the liquid so it becomes a vapor, which is then condensed into liquid form by cooling
Boiling point
Used as an index of purity in this experiment
Substance that evaporates readily or one that has measurable vapor pressure
Definition of volatile
Red-Colored Alcoholic Solution or Impure ethyl alcohol (CH3CH2OH)
120 cc of what was placed in the distilling flask
40 drops
How many drops were counted before changing the receiving flask?
To avoid high pressure that would cause explosion of the flask
Why shouldn’t the receiver be fastened to the condenser with a tightly fitted cork?
Distillate; Ethyl Alcohol (CH3CH2OH)
General name and compound name/formula of purified substance in the receiving flasks
Flammability Test
How was the presence of purified CH3CH2OH validated?
Distillation Apparatus
Another name for the distillation set up
Thermometer
Identify Apparatus (A)
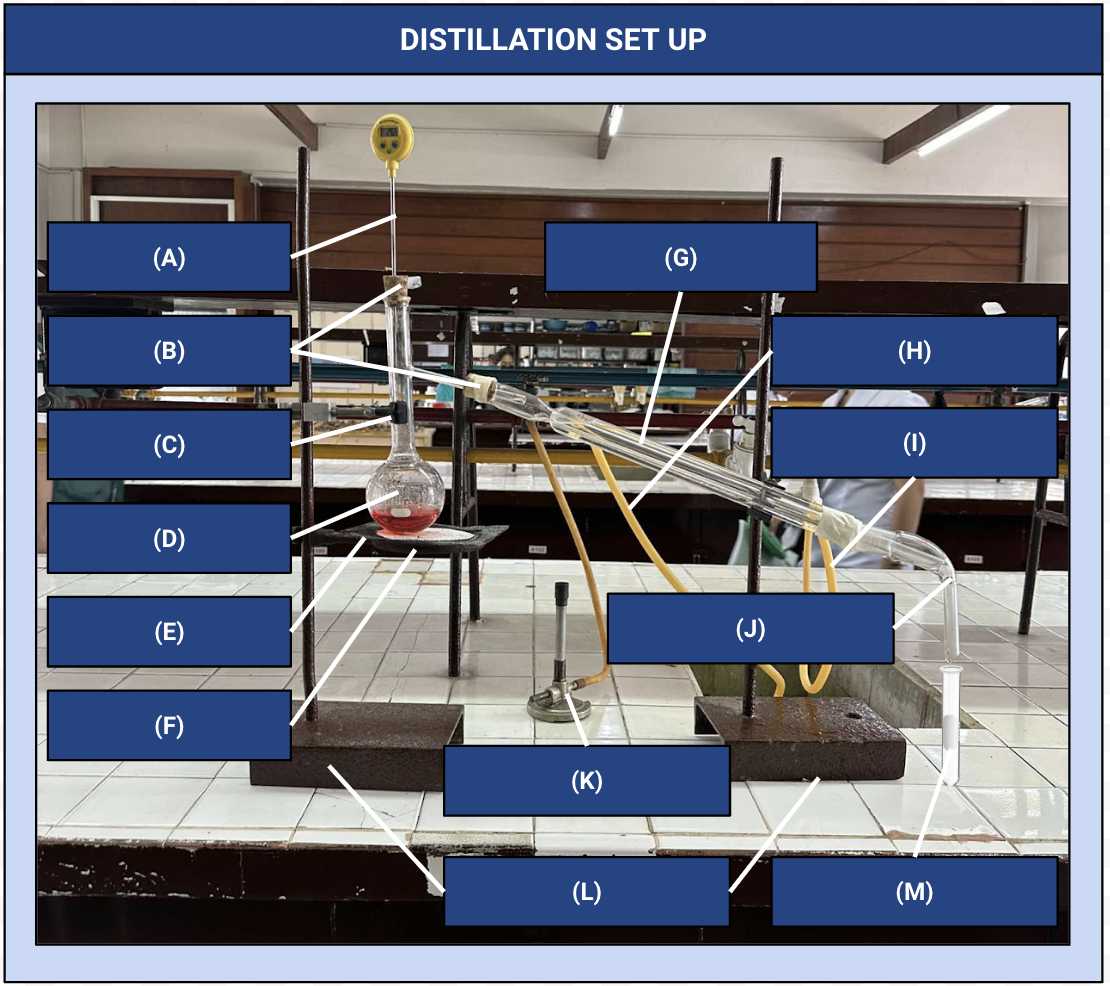
Corks
Identify Apparatus (B)
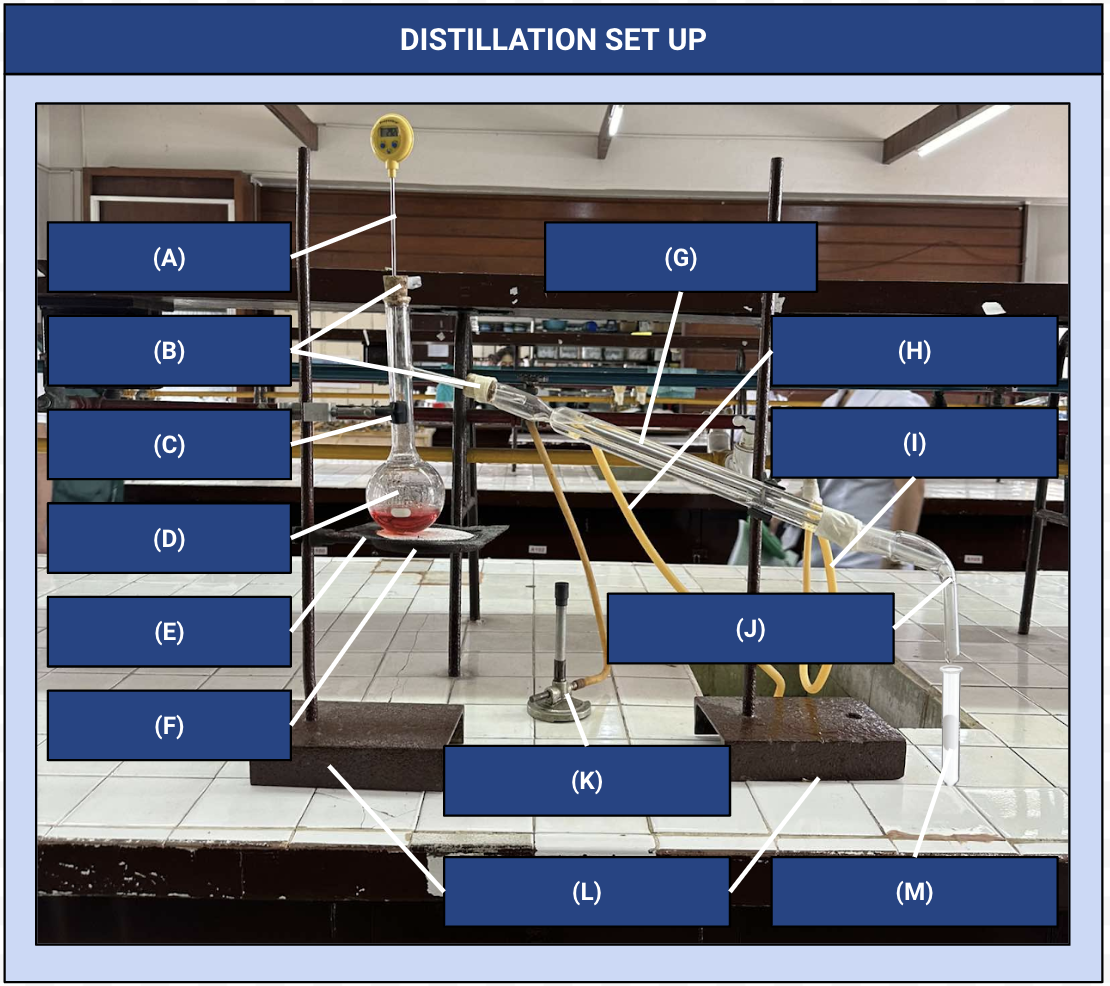
Iron Clamp
Identify Apparatus (C)
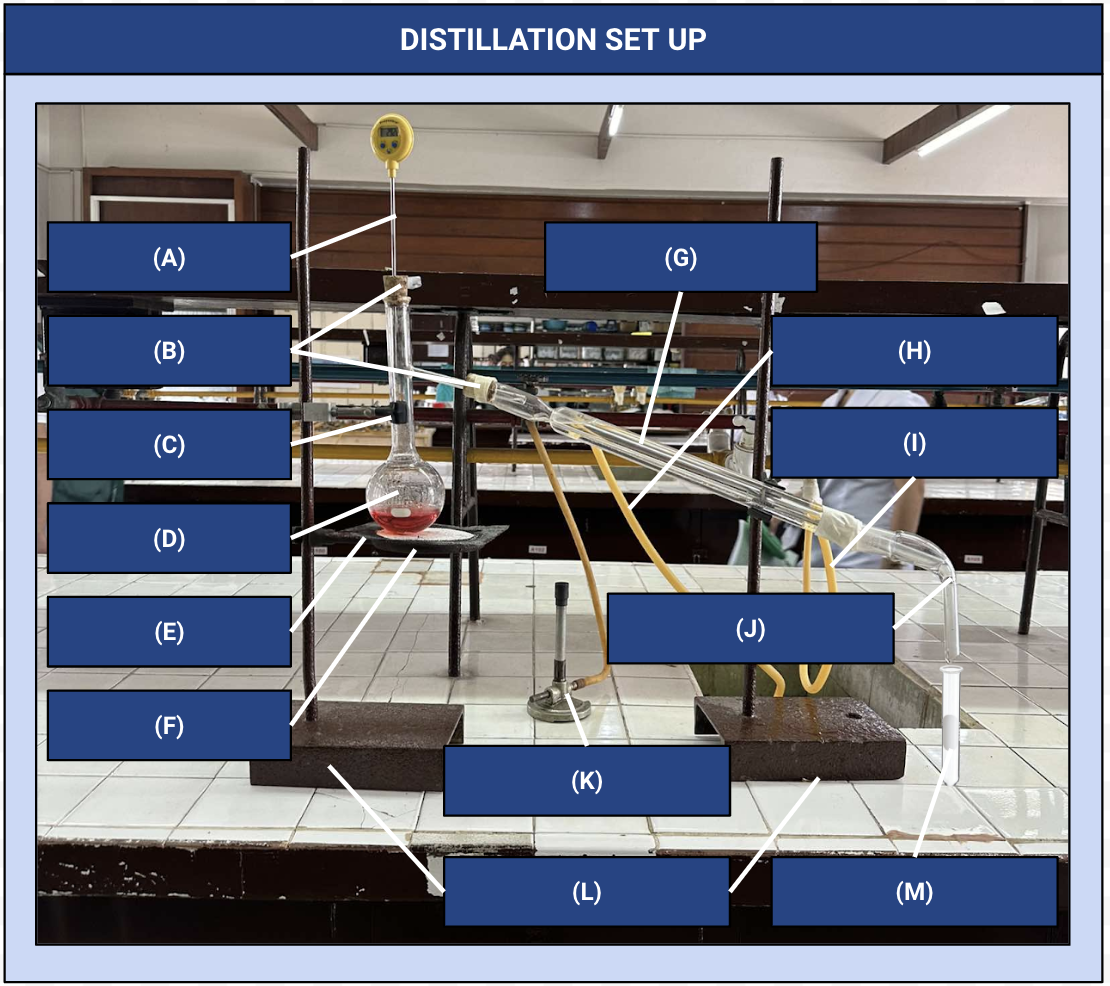
Distilling Flask
Identify Apparatus (D)

Wire Gauze
Identify Apparatus (E)
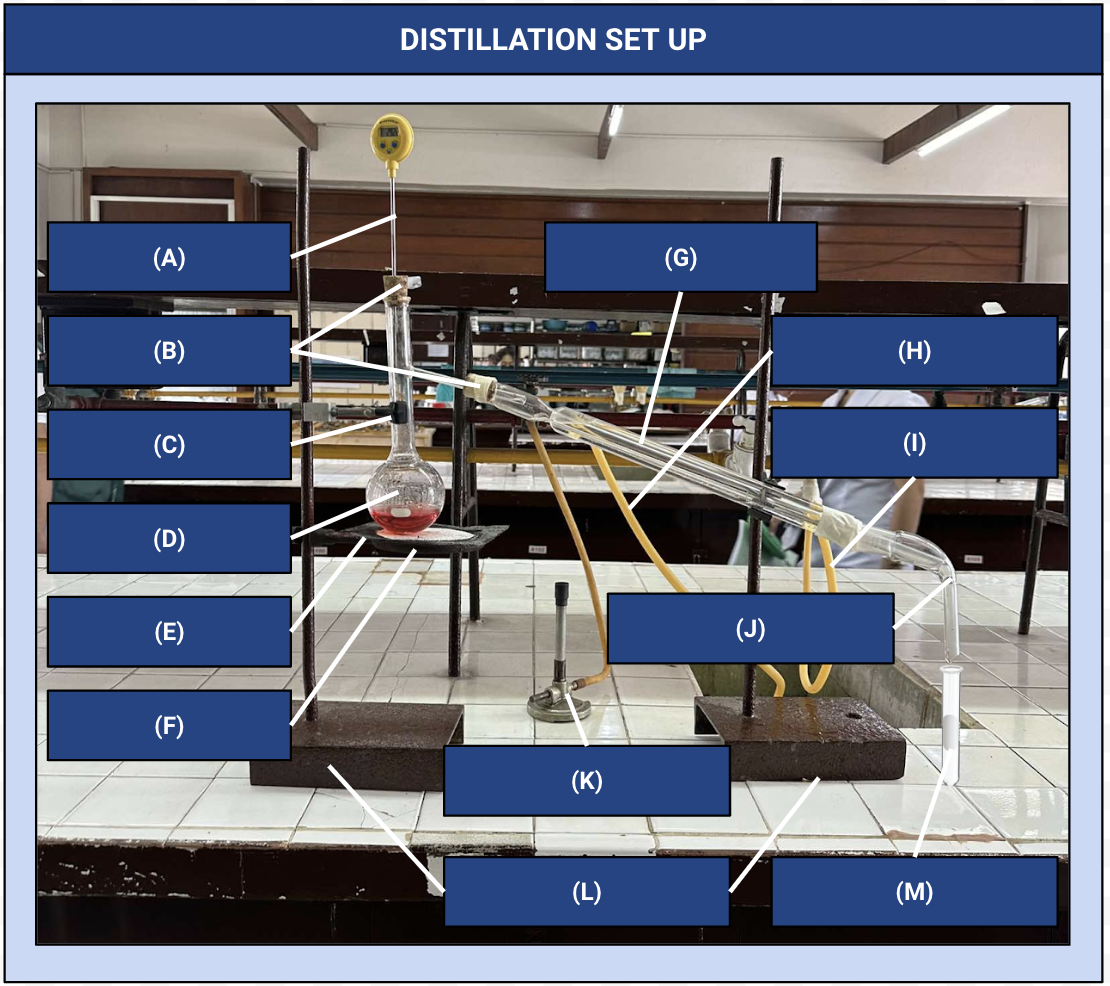
Iron Ring
Identify Apparatus (F)
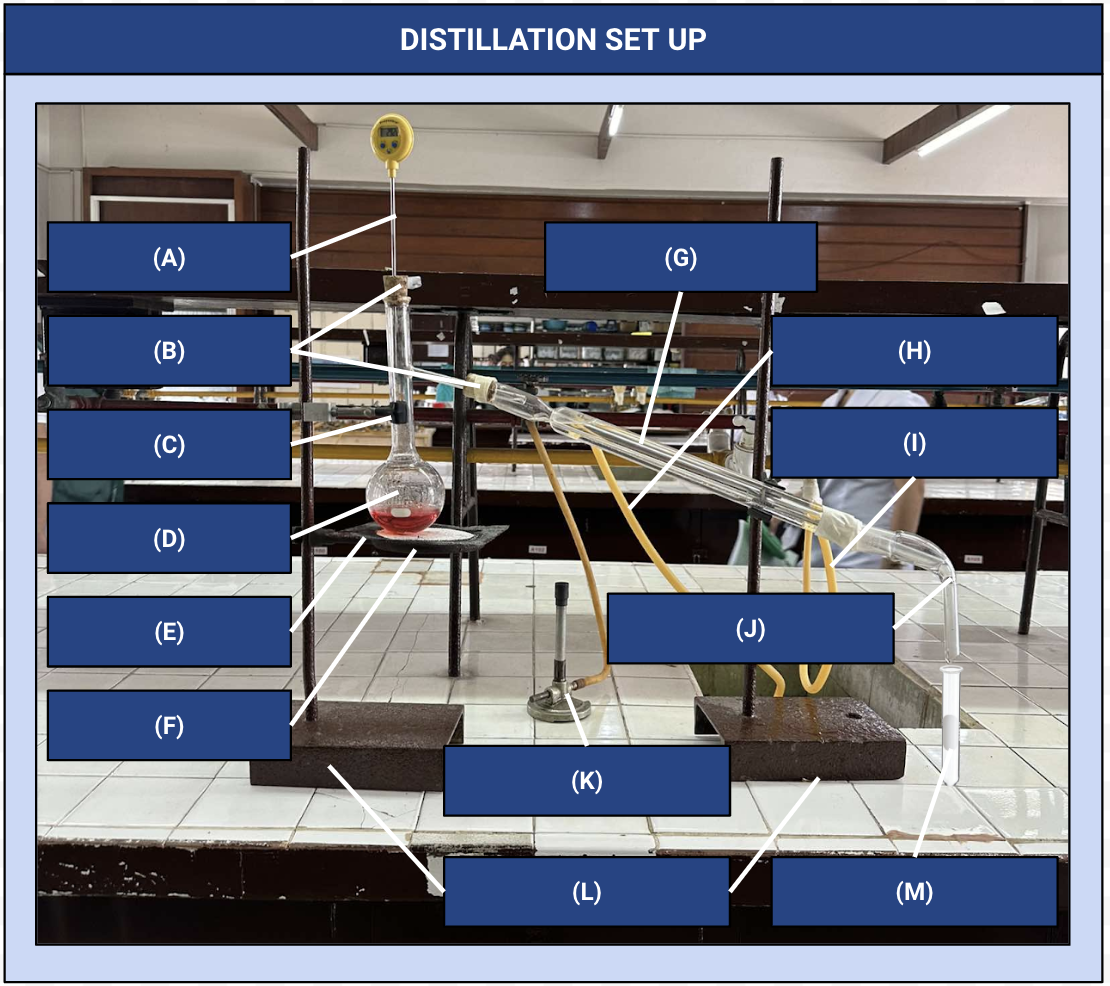
Condenser
Identify Apparatus (G)
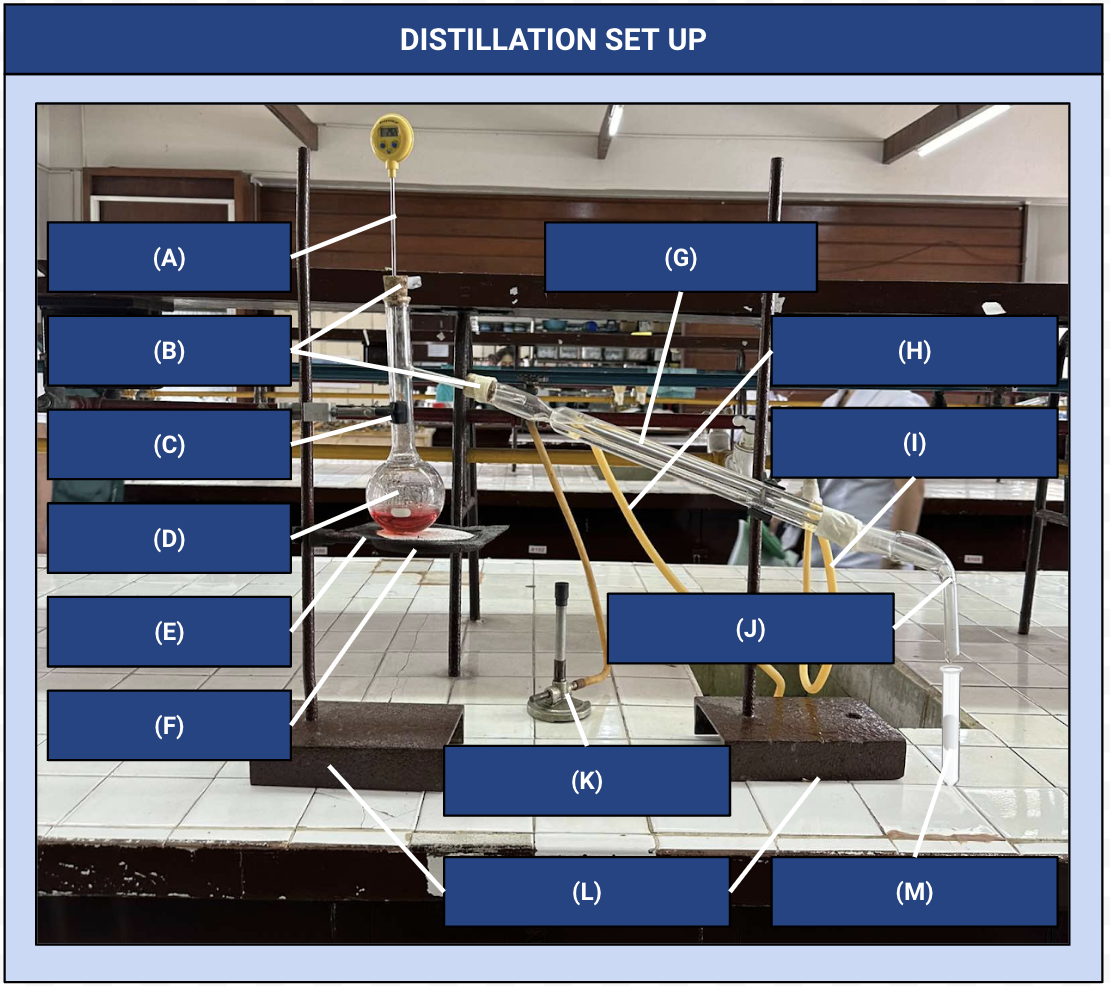
Water Outlet
Identify Apparatus (H)
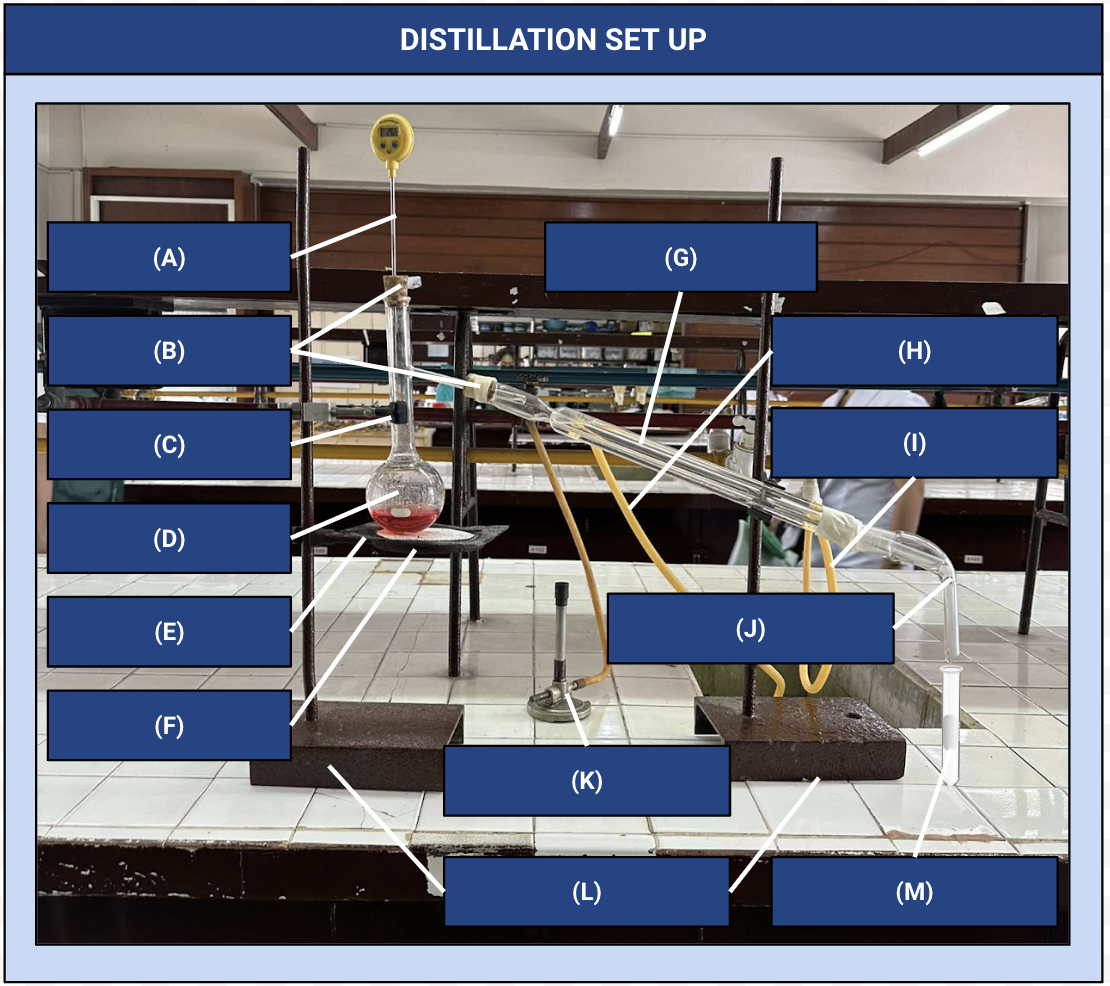
Water Inlet
Identify Apparatus (I)
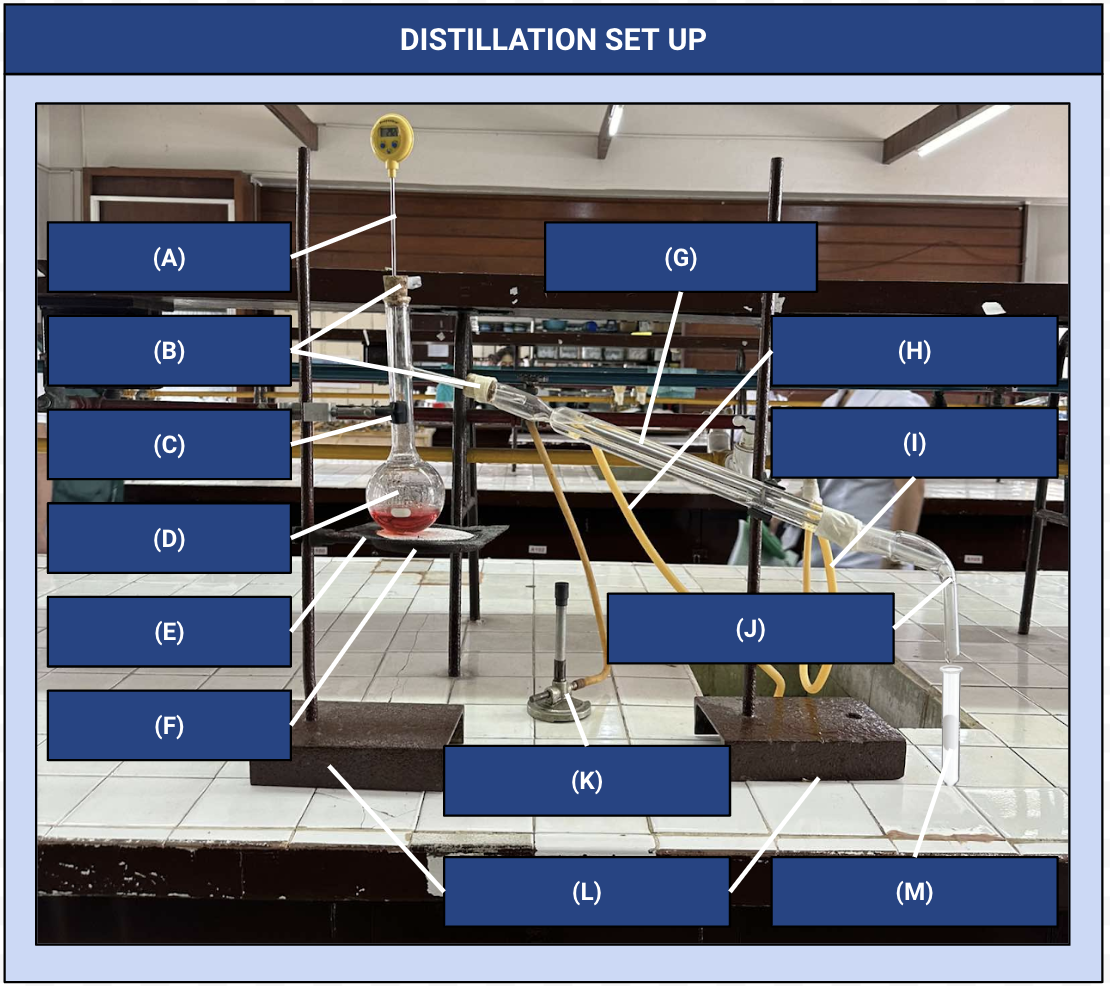
Receiver
Identify Apparatus (J)
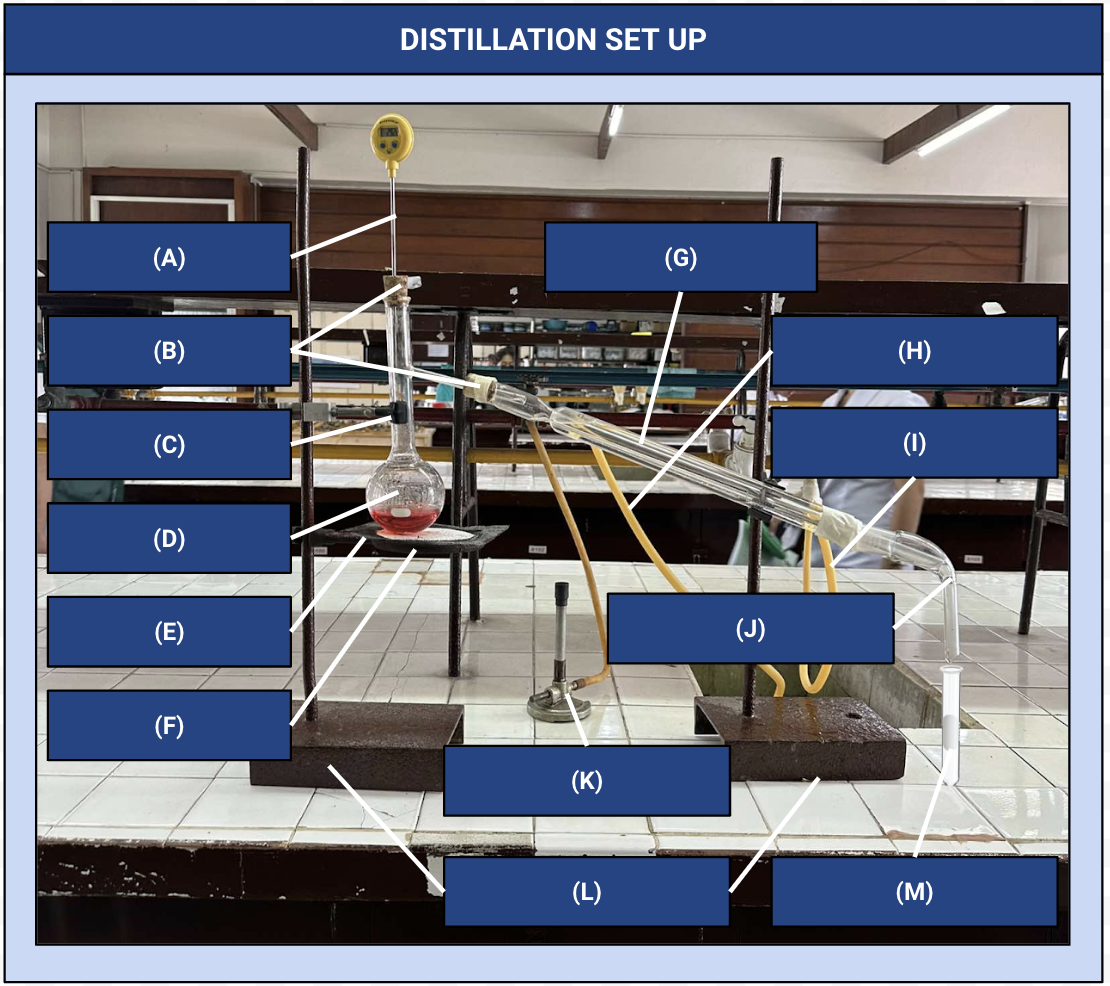
Bunsen Burner
Identify Apparatus (K)
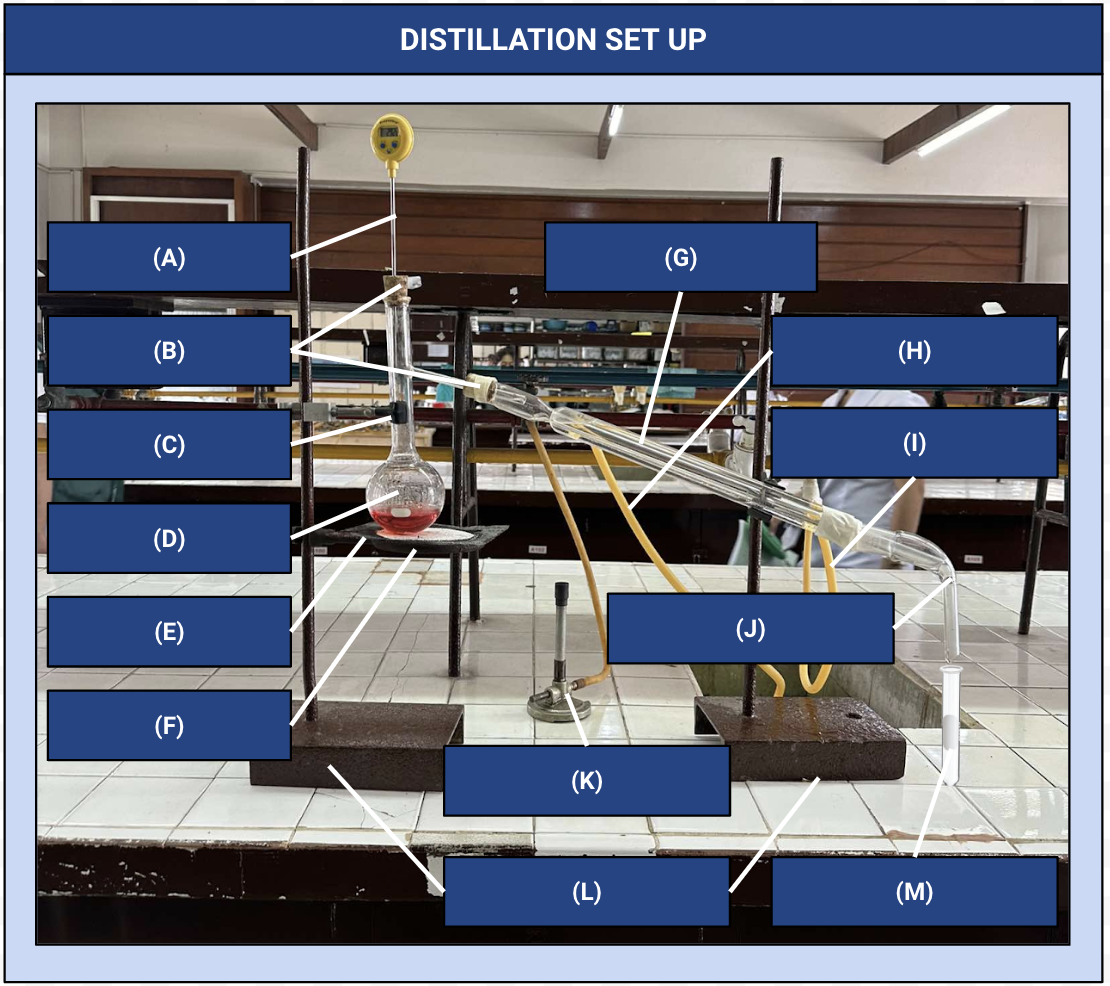
Holding Stands
Identify Apparatus (L)
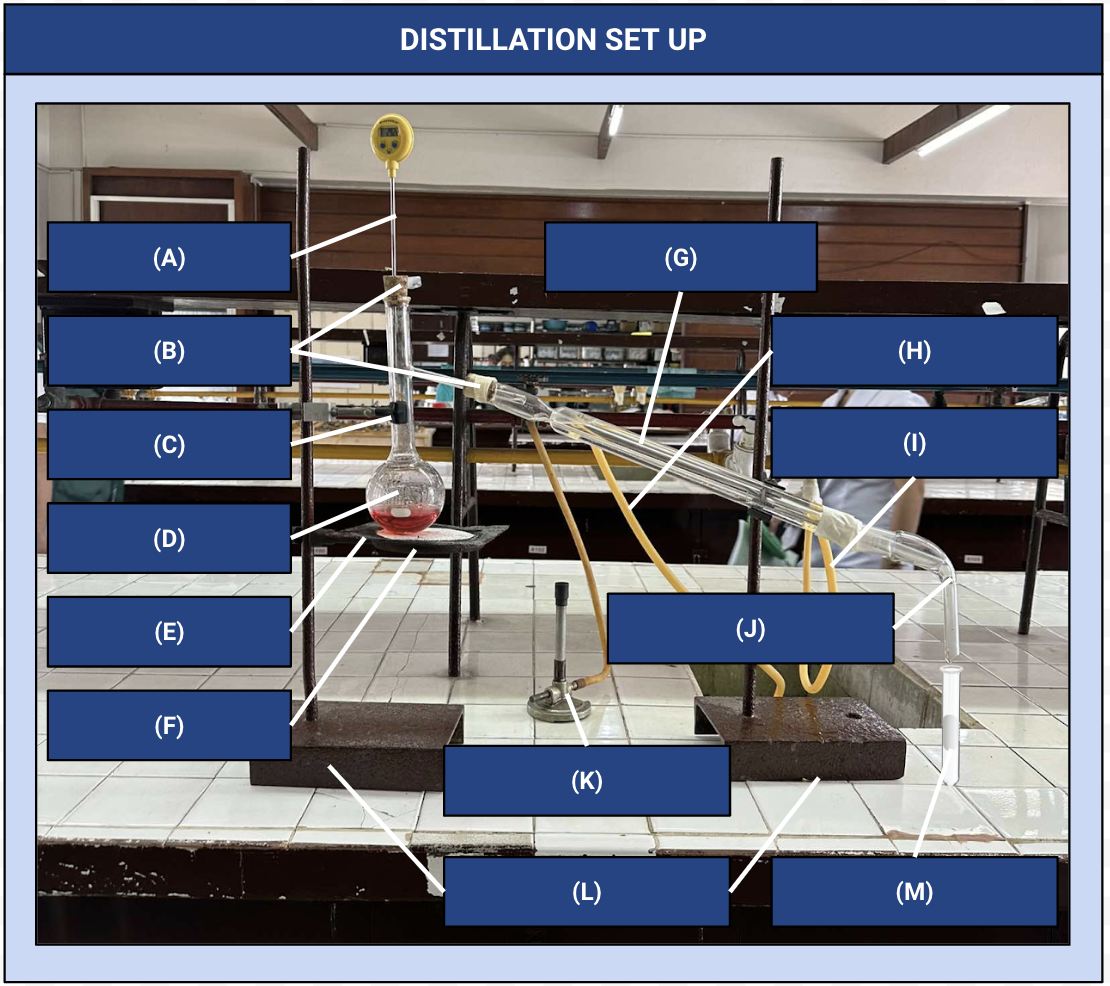
Receiving Flask
Identify Apparatus (M)
