Acid-Base Balance and Gases
1/76
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
77 Terms
Metabolic reactions → Acids and CO2
Arterial blood pH = 7.35 - 7.45
Maintained by Blood Buffers
Respiratory System
Renal System
Blood buffers:
First line of defense against pH changes
Weak acid + Conjugate base
Acts as an acid when base is added
Acts as a base when acid is added
Bicarbonate-Carbonic acid:
Principle plasma buffer
Responds in respiratory and renal acid-base regulation
H+ + HCO3- ←→ H2CO3 ←→ CO2 + H2O
Phosphate Buffers:
Renal Mechanisms
HPO2²-, H2PO4- (involved in exchange of sodium ion in the urine filtrate, plus H+)
Hemoglobin:
Major RBC buffer
Plasma Proteins:
Negatively charged, bind H+
Isohydric Shift:
H+ is shifted among acid-base pairs so that [H+] remains essentially unchanged
Isohydric shift: Plasma phase:
Some CO2 combines with H2O:
CO2 + H2O ←→ H2CO3 ←→ H+ + HCO3
H+ is buffered by plasma buffers, i.e., proteins
Isohydric shift: RBC phase:
Most CO2 enters RBC and combines with H2O
CO2 + H2O ←→ H2CO3 ←→ H+ + HCO3-
CO2 + H2O ←→ H2CO3
Carbonic anhydrase
H+ is buffered by oxyhemoglobin (HbO2-)
Oxyhemoglobin is transported to the tissues
Oxygen diffuses to the tissue cells
CO2 diffuses from the tissue into the RBC (deoxyhemoglobin)
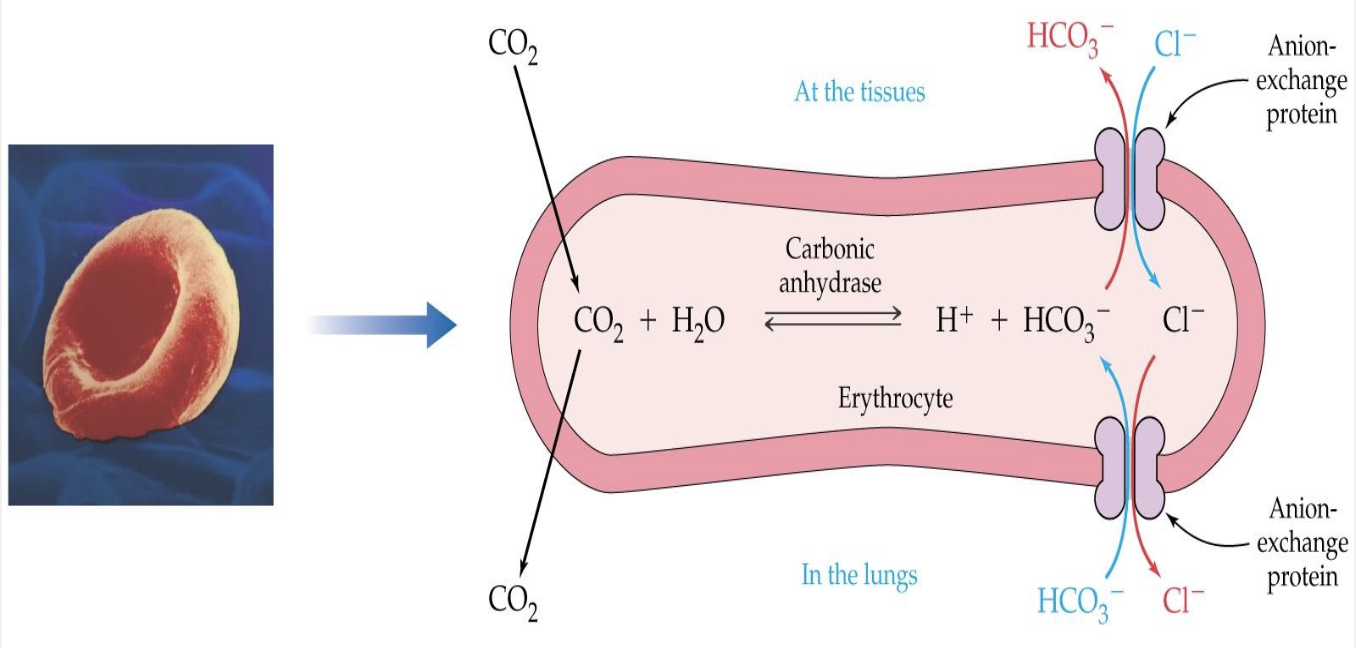
Isohydric and Chloride Shift — RBC
Chloride Shift (RBC):
HCO3- builds up in RBC, and diffuses into plasma
Cl- diffuses into RBC for electroneutrality
Renal Acid-Base Balance:
Final defense against changes in body pH
Excretes acid produced by metabolism
Excretes variable amounts of acid/base
Renal Acid-Base Balance Mechanisms:
Acid excretion
Na+ H+ exchange
H+ reacted with buffer base: PO3, NH3
HCO3- reabsorption (reclamation)
Vital to maintain plasma buffering
Generally proportional to Na+ reabsorption
Renal Acid Excretion: Na+ - H+ Exchange:
H+ secretion
Na+ reabsorption (active exchange)
K+ and H+ compete for exchange
Hyperkalemia
More H+ in RBCs, more K+ in plasma
Metabolic acidosis
Hypokalemia
More K+ in RBCs, more H+ in plasma
Metabolic alkalosis
Renal buffers: Phosphate
H+ + Na2HPO4 (dibasic Na phosphate) → NaH2PO4 (monobasic Na phosphate)
Excreted in urine
Renal buffers: NH3:
H+ + NH3 —> NH4+
Excreted in urine as ammonium salt, NH4Cl
Henderson-Hasselbalch Equation:
Bicarbonate-Carbonic acid Buffer System
Blood pH = 6.1 + log[HCO3-]/[H2CO3]
pH controlled by [HCO3-]/[H2CO3] ratio
Ratio normally 20/1
Increased ratio = Increased pH
Decreased ratio = Decreased pH
Bicarbonate (HCO3-) “Metabolic”
Kidney
Carbonic acid (H2CO3) “Respiratory”
Acid Base Imbalance: Respiratory disorders (CO2):
Increased CO2 (acidosis)
Decreased CO2 (alkalosis)
Renal system compensates
Acid Base Imbalance: Metabolic disorders (HCO3-):
Decreased HCO3- (acidosis)
Increased HCO3- (alkalosis)
Respiratory system compensates
Acid Base Imbalance: Acidosis:
Decreased pH
Decreased ratio
Make umeratory HCO3- smaller; OR make denominator (pCO2) larger
Acid Base Imabalance: Acidosis: Respiratory factor altered:
n [HCO3-]/INCREASED pCO2
Acid Base Imbalance: Acidosis: Metabolic factor altered:
DECREASED [HCO3-]/ n pCO2
Acid Base Imbalance: Alkalosis:
Increased pH
Increased Ratio
Make numerator (HCO3-) larger; OR make denominator (pCO2) smaller
Respiratory factor altered: pCO2
n HCO3- / decreased pCO2
Metabolic factor altered: HCO3-
Increased HCO3-/ n pCO2
Acid-Base Imbalance: Compensation:
Opposite factor moves in same direction as the primary problem to normalize pH (Bring back to 20:1 ratio)
Example: Primary problem is Respiratory factor: pCO2
n HCO3- / decreased pCO2
For compensation, HCO3- goes down to normalize ratio
pH becomes normal, BUT both factors now abnormal!!
Primary problem is the more abnormal result
Decreased HCO3-/ VERY DECREASED pCO2
Respiratory Compensatory Mechanisms:
Immediate, short-term, often incomplete
Respiratory Compensatory Mechanisms: Metabolic alkolosis:
Increased CO2 retention
Hypoventilate
Respiratory Compensatory Mechanisms: Metabolic acidosis:
Decrease CO2 (eliminate it)
Hyperventilate (“blow off” CO2)
Renal Compensatory Mechanisms:
Slower, (a few days to maximize), long-term, potentially complete
Renal Compensatory Mechanisms: Respiratory alkalosis:
Increased H+ retention
Decreased HCO3- retention
Renal Compensatory Mechanisms: Respiratory acidosis:
Decreased H+ retention
Increased HCO3- retention
Metabolic Acidosis:
1 degree Bicarbonate deficit
Decreased [HCO3-/[H2CO3] ratio → decreased pH
Increased anion gap
Metabolic Acidosis: Normochloremic type:
Endogenous acids:
Ketoacidosis, Lactic acidosis
Exogenous acids:
Isopropanol, Salicylate toxicity (Late)
Decreased acid excretion: Increase renal failure
Metabolic Acidosis: Hyperchloremic type:
Direct HCO3- loss
Increase in Cl- for electroneutrality
Metabolic Acidosis disorders:
Renal tubular acidosis (RTA)
Diarrhea
Metabolic Acidosis: Lactic Acidosis:
Glycolysis: Glucose → Pyruvate
Pyruvate converts to Lactate in anaerobic conditions instead of acetyl Co A
Lactic acidosis: Physiologic:
Strenuous exercise: increased blood lactate and pyruvate
Lactate/pyruvate ratio unchanged
Lactic acidosis: Pathologic:
Often leads to coma and death
Hypoxic: Shock, volemia, heart failure
Increased lactate/pyruvate ratio
Lactic Acid/Pyruvate Specimen:
Must minimize in vivo and in vitro glycolysis
Leads to false increased lactate; Pyruvate - unstable
Patient prep
Fasting, resting for >2 hours; No tourniquet/fish
Whole blood
Collect in heparnized tube, on ice, gray top
Transfer blood to TCA (trichloroacidic acid) or perchloric acid tube
Plasma
Collect in NaF, K oxolate tube, gray top, on ice
Separate from cells within 15 minutes
Metabolic Acidosis Compensation Mechanisms:
Respiratory
Blow off CO2 to raise ratio toward normal
Renal
Increased acid excretion
Increased HCO3- reabsorption (reclamation)
Metabolic Acidosis: Lab findings:
Decreased pH, decreased HCO3-
After compensation: decreased pCO2
Other findings: Cl- might be increased (Hyperchloremic)
Metabolic Alkalosis:
1 degree bicarbonate excess
Increased [HCO3-]/[H2CO3] ratio → increased pH
Metabolic Alkalosis: Disorders:
Antacid or bicarbonate overdose
Increased H+ excretion/loss
Prolonged vomiting, nasogastric suctioning
Increased K+ depletion
Increased Na+ retention (hyperaldosteronism)
Diuretics
Metabolic Alkalosis: Compensation:
Respiratory
Increased CO2 retention
Hypoventilation
Renal
H+ retention
Decreased HCO3- retention
Metabolic Alkalosis: Lab Findings:
Increased pH, increased HCO3-
After compensation: increased pCO2
Other findings: alkaline urine pH
Respiratory Acidosis:
1 degree CO2 excess (Hypercapnia)
Decreased [HCO3-]/[H2CO3] ratio → decreased pH
Hypoventilation
Respiratory Acidosis: Disorders:
Chronic obstructive pulmonary disease (COPD)
Asthma, pulmonary emphysema
Chemoreceptor depression
Drugs, i.e. heroin, morphine
Rebreathing exhaled air
Respiratory Acidosis: Compensation mechanisms:
Renal: Increased acid excretion, increased bicarb
Respiratory: blow off CO2
Respiratory Acidosis: Lab findings:
Decreased pH
Increased pCO2
Decreased O2
After compensation: Increased HCO2- (retained since kidney is primary compensation)
Other findings: Cl- may be decreaed
Respiratory Alkalosis:
1 degree CO2 deficit (Hypocapnia)
Increased [HCO3-]/[H3CO3] ratio → increased pH
Hyperventilation
Respiratory Alkalosis: Disorders:
Phsychogenic, i.e. anxiety, hysteria
Hyperstimulation of respiratory center
Hypoxia
Drugs, i.e. salicylate toxicity (Early)
Respiratory Alkalosis: Compensation mechanisms:
Renal: Increased H+ retention, decreased bicarb. retention
Respiratory: Increased CO2 retention
Respiratory Alkalosis: Lab findings:
Increased pH, decreased pCO2
After compensation: decreased HCO3-
Other findings: Alkaline urine pH
Oxygen Significance: Increased pO2
O2 administration
Hyperventilation
Oxygen Significance: Decreased pO2
Hypoxemia
Hypoventilation
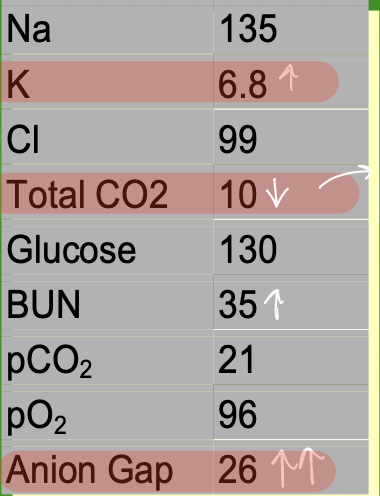
Case: A 13-year-old male was brought to the
emergency room in a comatose state.
His mother stated that he was nauseated
earlier that day. Upon physical
examination, it was noted that the patient
was breathing deeply and rapidly, his
breath had a fruity odor, and the skin and
mucous membranes were dry.
Pt has diabetic ketoacidosis
Due to increased glucose, acetone in urine
BUN is increased in DM due to dehydration, since urea moves with water and is retained, leading to a buildup of NPNs
Loss of body water is indicated by osmolality and decreased Na+
Glycosuria is due to being past the renal threshold
Metabolic acidosis
ABG and pH test results:
pH decreased
pCO2 decreased
pO2 increased
pH and Blood Gas Analysis: Measured analytes include:
pH, pO2, and pCO2
pH and Blood Gas Analysis: Derived parameters include:
H2CO3, HCO3-, Base excess (BE), TCO2, %SO2
H2CO3 directly related to pCO2
H2CO3 calculation:
pCO2 × 0.031
HCO3- calculation:
Blood pH = 6.1 + log[HCO3-]/[pCO2 × 0.031]
Titratable Base Excess (BE):
Estimates Metabolic component of Acid-Base disorder
Positive values (base excess)
Suggests metabolic alkalosis
Negative values (base deficit)
Suggests metabolic acidosis
Numerical values indicate theoretical amount of acid/base (mmol/L) to correct blood pH
Less given in practice, b/c of compensation
pH and Blood Gas Specimen: Whole blood:
Arterial (ABG), venous, capillary
pH and Blood Gas Specimen:
Heparinized syringe!
Anaerobic technique
Air exposure causes: increased pH, increased pO2, decreased pCO2
Mix well to avoid clots
Transport on ice, analyze immediately (<30 min)
Excessive metabolism causes:
Decreased pH, decreased pO2, increased pCO2
ABG Reference ranges: pH:
7.35-7.45
ABG Reference ranges: pO2:
80-90 mmHg
ABG Reference ranges: pCO2:
35-45 mmHg
ABG Reference ranges: O2 saturation:
95-99%
pH and Blood Gas Analysis:
Whole blood mixed well
Sample aspirated, warmed to 37 C
Electrodes protrude into sample chamber
pH and ABG Electrodes:
H+ gas
pH Reference electrode: calomel/sat.KCl or Ag-AgCl2/sat. KCl
Salt bridge (contracts sample)
pCO2 electrode: gas permeable membrane, e.g. silicone
Modified pH electrode
pO2 electrode: gas permeable membrane, i.e. polypropylene
Platinum cathode → Current (Amperometric)
ABG Instrument Calibration:
Frequent verification (every 30 minutes)
pH
pH standards (High + Low)
pO2 and pCO2
pO2 and pCO2 gases (high + low)
ABG Errors: Pre-analytical:
Sample collection, transport, poor mixing before sampling
Patient temperature
Need actual temperature to correct result
Especially important for accurate pO2
ABG Errors: Calibration:
Wrong set points
ABG Errors: Instrument Temperature control (> ± 1 C)
pO2 electrode
ABG Errors: Dirty sample chamber/sample path:
Flushed between samples to avoid blood/fibrin clot build-up
Interferes with electrode contact (including salt-bridge) with calibrators, samples
Incomplete specimen sampling
ABG Instrument Troubleshooting: All analytes out-of-control:
Recalibrate
ABG Instrument Troubleshooting: All analytes out-of-control and calibration fails:
Check sample chamber/sample path, electrode for dirt, blood build-up, or clots
Check calibrator materials/set points
ABG Instrument Troubleshooting: Specific analyte(s) out-of-control:
Check specific electrode(s), replace membranes as needed
pO2 flucuation
Check instrument temperature
Oximetry: Co-oximeter:
Measures oxygen saturation (SO2), carbon monozid (COHb), methemoglobin (MetHb)
Spectrophotometry
Characteristic absorption wavelengths of various hemoglobin species
Oximetry: Pulse oximeter:
Attaches to finger, toe, or earlobe
Measures SO2 trends
Spectrophotometry
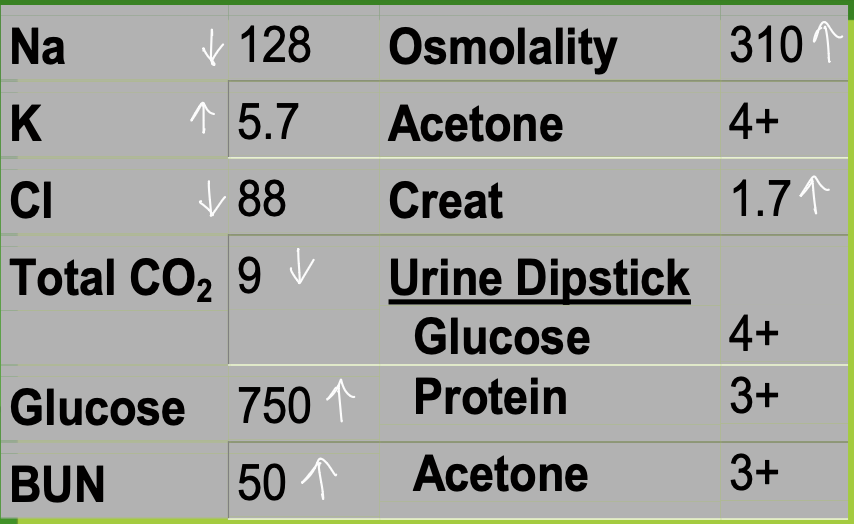
Case 2: A 5-year-old girl was admitted to the ER in a
comatose state. The following laboratory
results were found:
Decreased total CO2
Increased Anion Gap
Ethylene glycol poisoning