2.1 - Intro to Electrolysis
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
18 Terms
What is electrolysis?
breaking down an ionic compound into its elements using electricity
Where does electrolysis occur?
in an electrolytic cell
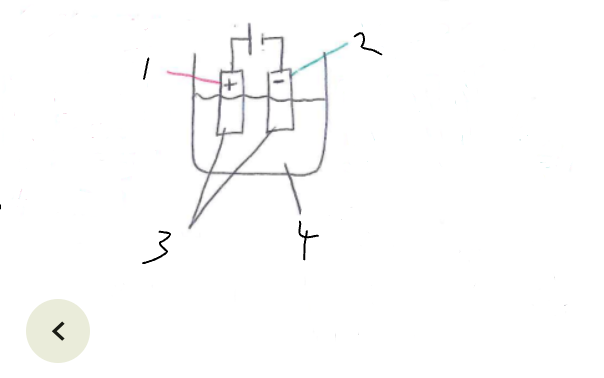
What part of the electrolytic cell is 1
anode
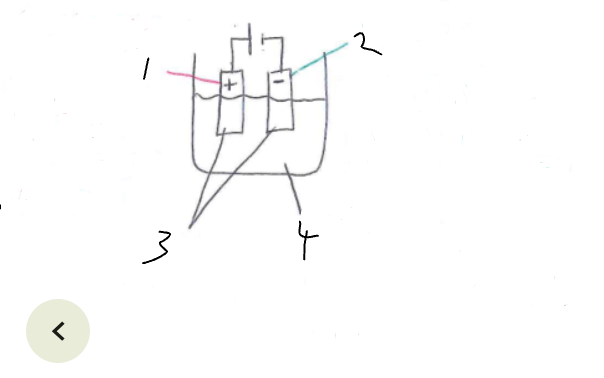
What part of the electrolytic cell is 2
cathode
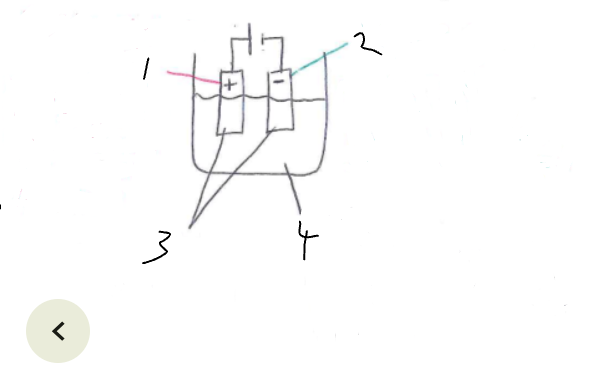
What part of the electrolytic cell is 3
electrodes
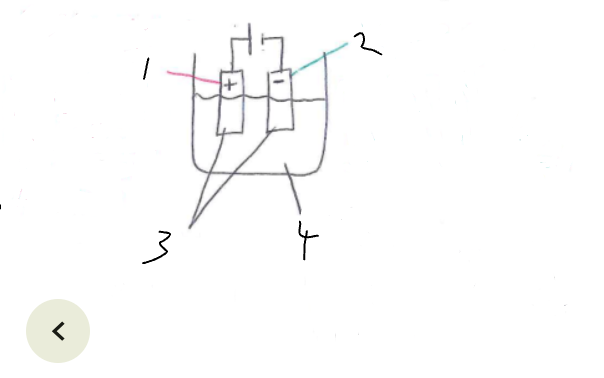
What part of the electrolytic cell is 4
electrolyte
What is an anode?
positive electrode
What happens at the anode?
negative ions are attracted to the anode and lose electrons
What is a cathode?
negative electrode
What happens at the cathode?
positive ions are attracted to the cathode and gain electrons
What is special about electrodes?
they are inert (unreactive)
What is electrolyte?
molten ionic compounds or solutions that conduct electricity
What is oxidation?
loss of electrons
Where does oxidation occur?
at the anode
What is reduction?
gain of electrons
Where does reduction occur?
at the cathode
What is an easy way to remember oxidation and reduction?
OIL RIG = Oxidation is Loss, Reduction is Gain
What is an easy way to remember which ion goes to which electrode?
PANIC = Positive Anode, Negative Is Cathode