BSCI 170 - Intro to Cellular Respiration & Fermentation, Aerobic Respiration, and Oxidative Phosphorylation
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
Oxidation of Cellular Fuel
Each electron transfer reaction is __________
Electrons ____ energy with each transfer
Electrons in H2O have ____ (potential) energy than they did in glucose
Released free energy drives ___ ________
exergonic, lose, less, ATP synthesis
Stepwise Energy Harvest
Is energy from glucose released at once?
How is glucose combusted?
Electrons (and protons) are ________ ____ and _________ to another molecule (electron carrier).
no
in a series of small, enzyme-catalyzed steps
stripped away, transferred
Nicotinamide Adenine Dinucleotide
Derivative of Niacin
Oxidized form, ____, serves as an important ________ ________ during cellular respiration
What is the reduced form?
NAD+, electron acceptor
NADH
Cellular Respiration
Respiration is the cumulative effect of 4 separate events. What are they?
Glycolysis (lysis of glucose)
Pyruvate Oxidation and Citric Acid Cycle (oxidation of carbon and salvaging of high-energy electrons)
Electron Transport (stripping energy from the electrons)
Oxidative Phosphorylation (using the energy from the electrons to synthesis ATP)
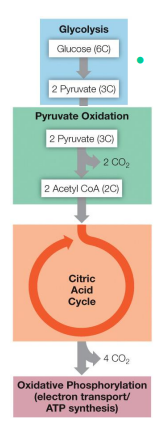
Without the process of cellular respiration, the addition of oxygen to H2 would result in an _________ _______.
explosive release
Oxidation of Glucose
C6H12O6 + 6O2 —> 6CO2 + 6H2O + Energy
Oxidation of glucose means electrons (and protons) are removed from _______ and transferred to ______.
The first step in this complex, multistep process is ________.
glucose, oxygen, glycolysis
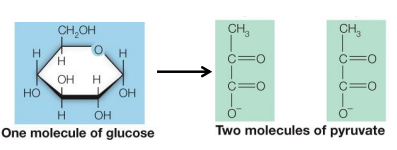
Glycolysis
Glycolysis means _____ _________
glyco = ________ __ _____ & lysis = __ ______
occurs in the _______ of __________
one six-carbon sugar (_______) is ________ to ___, _____-______ __________ (________)
sugar splitting, relating to sugar, to unbind, cytosol, eukaryotes, glucose, oxidized, two, three carbon molecules, pyruvate
Fermentation
There are many different fermentation pathways
All have two common purposes. What are they?
What is reduced in fermentation?
Oxidize NADH to regenerate NAD+
Allow glycolysis to continue
Pyruvate (or a derivative of pyruvate) is reduced
Lactate Fermentation
In fermentation, ________ is reduced and ____ is oxidized
Pyruvate + NADH + H+
pyruvate, NADH
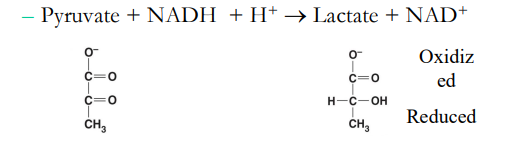
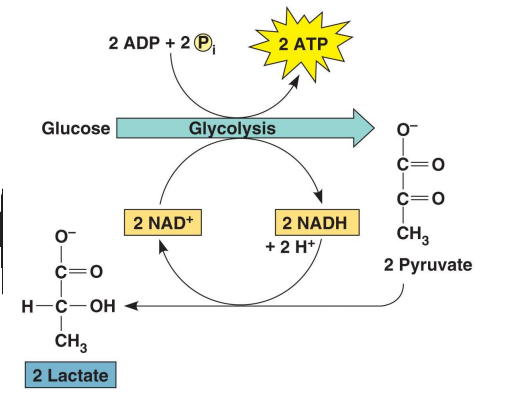
What is pictured in this image?
lactate fermentation
Lactate Fermentation
No release of ___
No consumption of __
No consumption of ___
Occurs in vertebrate skeletal muscles during __________ _______
Lactate build-up contributes to the burning feeling of ______ _______
CO2, O2, ATP, anaerobic workout, active muscles
Lactate Fermentation in Food
Use bacteria to perform desirable lactic acid (lactate) fermentation (undesirable = ________)
Important for production of yogurt, pickles, sauerkraut, kimchi, sour cream, sourdough bread, kombucha, etc..
Kombucha SCOBY (Symbiotic Culture of Bacteria and Yeast (kombucha = lactate and alcohol fermentation)
spoilage
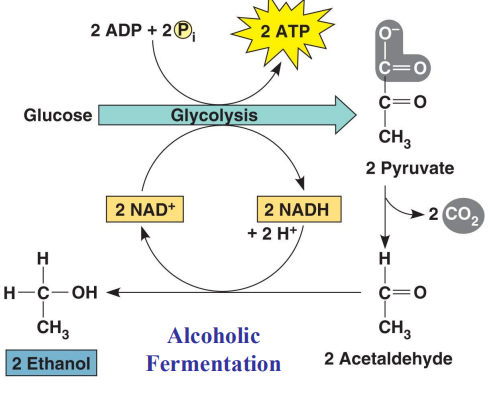
Alcoholic Fermentation
Pyruvate is converted into ____________ and ______ _______
Acetaldehyde is _______ to ________
Common process in microorganisms such as Brewer’s Yeast
acetaldehyde, carbon dioxide, reduced, ethanol
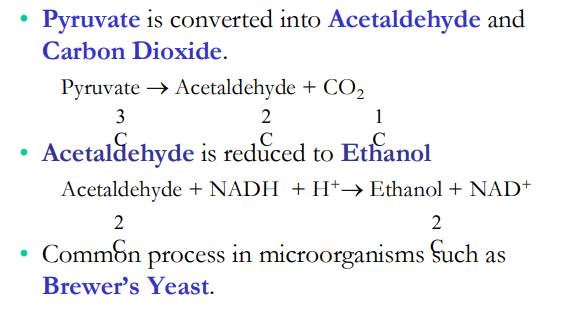
Alcoholic Fermentation with Yeast
In alcohol fermentation, pyruvate is converted to ethanol in two steps
The first step _________ ___
The second step ________ _______
Alcohol fermentation by yeast is used in brewing, winemaking, and baking
releases CO2, produces ethanol
Ethanol Toxicity
Everyone knows that ethanol is toxic.
Too much of a good thing can have lots of negative consequences
Reason: Think _________ __________
reversible reactions
Ethanol Toxicity
Too much ethanol leads to ___________ of _____
Too much acetaldehyde is _____
metabolic breakdown leads to ________, etc.
dehydration, cells, toxic, headaches
Alcohol Toxicity
Drinking bad moonshine can be highly dangerous because of production of methanol
Methanol (CH3OH) is more toxic than _______ (CH3CH2OH)
Why?
ethanol, due to reversible reactions
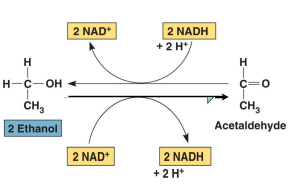
Moonshine Toxicity
Methanol and ethanol are substrates for the same enzyme: _______ ______________
Ethanol (2C alcohol) ←→ Acetaldehyde (2C aldehyde)
Methanol (1C alcohol) ←→ Methaldehyde (1C aldehyde)
alcohol dehydrogenase
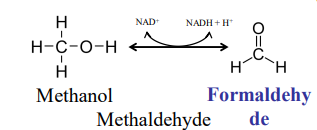
Methanol Toxicity
Another name for methaldehyde: ____________
Formaldehyde = ________ _____
formaldehyde, embalmer’s fluid
Formaldehyde Toxicity
Formaldehyde converts to ______ ____
In humans, ______ ____ leads to __________ _________ at low concentrations
formic acid, formic acid, permanent blindness
Problem at the End of Glycolysis
Glycolysis is ____ but is not an efficient use of the _________ ________.
After glycolysis and fermentation, more than 90 percent of the energy is still trapped in ______ _______ __________.
________
_______
________
Etc.
fast, starting material, reduced organic molecules, pyruvate, lactate, ethanol
Aerobic Respiration
So far no ______ has been used
In the presence of ______, the _______ organics can be completely ________.
This is Solution 2 for regenerating ____
oxygen, oxygen, reduced, oxidized, NAD+
After Glycolysis
Most of the energy (90 percent) in glucose is still ________.
In the absence of O2: ____________
allows regeneration of ____
allows __________ to continue
untapped, fermentation, NAD+, glycolysis
In the Presence of Oxygen
Another fate: ________ __________
occurs in the ______ __ ___ ____________
complete oxidation, matrix of the mitochondria
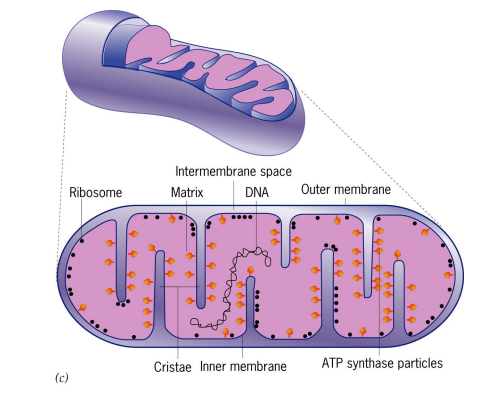
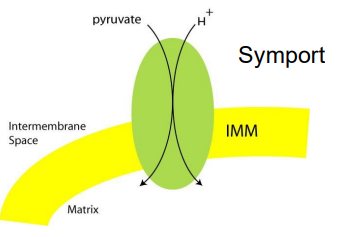
Step 1: Getting Pyruvate into the Mitochondrial Matrix
Pyruvate must pass through both the outer and inner ______________ _________ to get into the ______
Pyruvate moves passively across the outer membrane via _____ (membrane protein)
Pyruvate is actively transported across the inner membrane into the matrix via a ___-________ _____________
mitochondrial membranes, matrix, porin, H+ pyruvate cotransporter
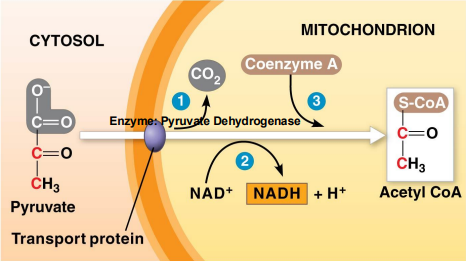
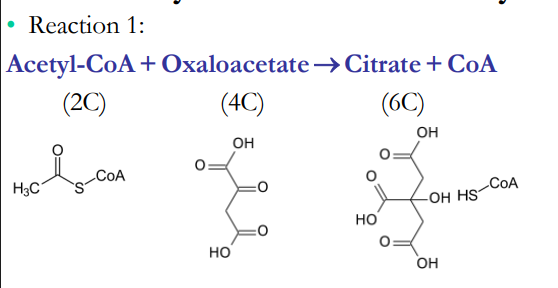
Once in the Matrix, Pyruvate is Decarboxylated
For your reference
N/A
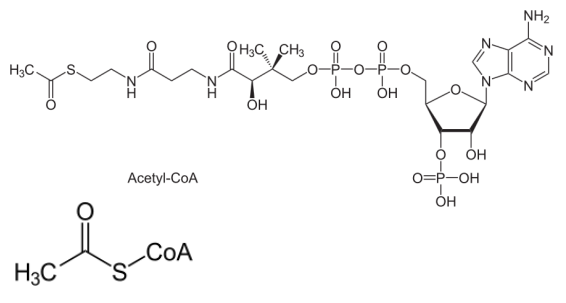
Acetyl-CoA is an important coenzyme in ____________ and _____ ____ ___________
carbohydrate, fatty acid metabolism
Acetyl-CoA
Acetyl-CoA is a ________ in the ______ ____ _____
We tend to think of metabolic pathways as being linear, but a cycle occurs when the product of the final reaction is also a _________ in the first reaction
reactant, citric acid cycle, reactant
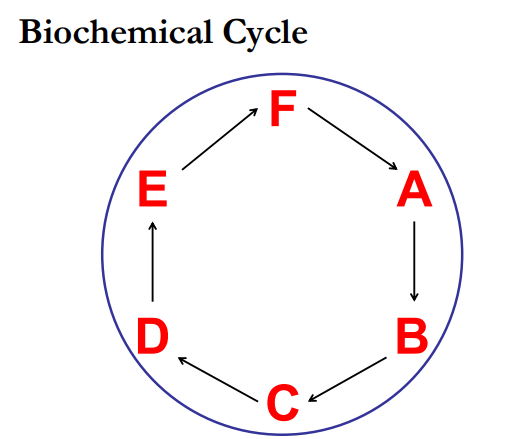
Biochemical Cycles
There is no ________ point or ______ point to a metabolic cycle
Intermediates can enter the cycle at any point.
starting, ending
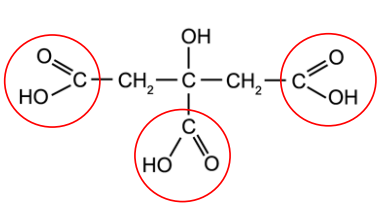
Citric Acid Cycle
Biochemical cycles often named for the first ____________ product
Citric acid (or citrate)
6-Carbon organic acid
3 Carboxyl groups (COOH)
recognizable
Citric Acid Cycle
Also known as the Tricarboxylic Acid (TCA) Cycle, the Citrate Cycle, and the Krebs Cycle (after Hans Krebs)
For your reference
N/A
Is pyruvate decarboxylation a part of the citric acid cycle?
no
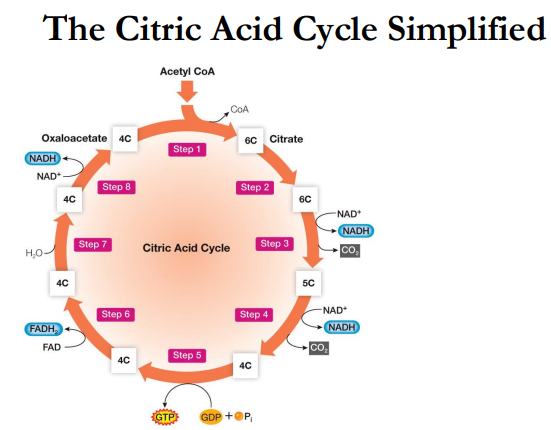
Summary of the Citric Acid Cycle
N/A
Summary of the Citric Acid Cycle
During the next 7 reactions there are:
2 _____________ (removal of 2 ___)
4 _________ (along with 4 ________)
3 ____ —> 3 ____ + 3 __ (6 ________)
1 ___ —> 1 _____ (2 ________)
1 _________-______ _______________ (___)
All to regenerate the starting material: ____________ (__)
Remember: ___________ produces 2 _________ for each molecule of ________.
Oxidizing both pyruvates from glycolysis requires progression through __________ _________ and the _____ ____ _____ ___ _______
decarboxylations, CO2, oxidations, reducations, NAD+, NADH, H+, electrons, FAD, FADH, electrons, substrate-level phosphorylation, ATP, oxaloacetate, 4C, glycolysis, pyruvates, glucose, pyruvate oxidation, citric acid cycle, 2 times
Coenzyme and CO2 Accounting in the Matrix
For each entering glucose molecule:
__ carbons generating __ pyruvates
__ pyruvate oxidations (w/ decarboxylation)
__ CO2
__ NADH
__ turns through the ______ ____ _____
__ CO2
__ NADH
__ FADH2
6, 2, 2, 2, 2, 2, citric acid cycle, 4, 6, 2
What has been achieved by the end of the citric acid cycle?
All __ carbons in glucose have been fully _________ to ___
__ ATPs generated by _________-_____ _______________
__ from __________
__ from ___ turns of the ______ ____ _____
>90% of remaining energy is in the form of “high-energy” electrons stored in ________ __________
____ & _____
6, oxidized, CO2, 4, substrate-level phosphorylation, 2, glycolysis, 2, 2, citric acid cycle, reduced coenzymes, NADH, FADH2
Electron Transport
High-energy electrons are removed from the _______ __________
Their energy is slowly extracted through a stepwise series of _________ ___________ and __________ ______.
electron transport chain (ETC)
reduced coenzymes, exergonic oxidation, reduction steps
Respiratory Complexes
__ large multiprotein complexes embedded in the ____ ____________ ________ comprise the ___
Respiratory complexes I-IV
Also __ small, mobile ________ _________
Coenzyme Q and Cytochrome C ____ _________ between complexes
4, inner mitochondrial membrane, ETC, 2, electron carriers, move electrons
Electron Transfer
Each electron transfer represents an __________-__________ reaction
Each electron transfer is _________
Each successive carrier binds electrons with a ______ affinity than the previous one
each successive carrier is more ___________ than the previous one
Electrons ____ energy as they move away from ____ or _____.
oxidation reduction, exergonic, higher, electronegative, lose, NADH, FADH2
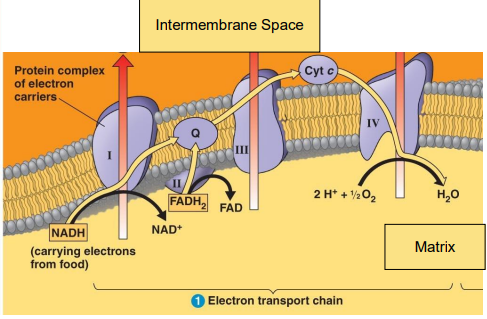
Oxidation of NADH and FADH2
For your reference
N/A
What is coenzyme Q (CoQ)?
a lipid-soluble, mobile electron carrier
CoQ accepts electrons from Complex I & Complex II and delivers them to Complex III
Also used as a nutritional supplement
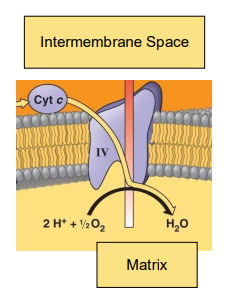
Reduction of Oxygen
The reduction of O2 to ___ in the final step of electron transport is the entire reason we need to breathe oxygen to survive
H2O
Accounting Time: End of ETC
All of the carbons in glucose have been oxidized to ___
By the end of ______ ____ _____
All of the electrons have been _______ to the energy level of _____
CO2, citric acid cycle, reduced, water
Coupling of ATP Synthesis and the ETC
By the 1960s, the basic functions of mitochondria were well established. What are they?
What was not known about the functions of mitochondria?
primary oxidation center of eukaryotic cells
oxidation of carbohydrates and fatty acids
electron transport chain (ETC)
ATP synthesis
What wasn’t known: How these were connected
ATP Synthase Complex
Another large, multiprotein complex embedded in the _____ _________ ________
inner mitochondrial membrane
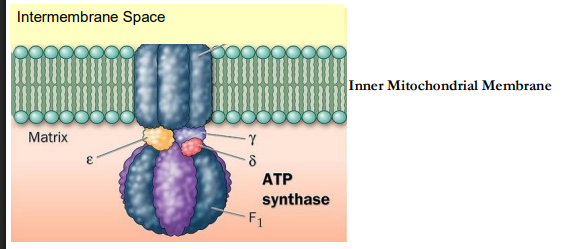
Coupling Factor F0F1
What is F0?
What is F1?
H+ channel
ATPase
ATP Synthase Complex
Functions like an active H+ transport protein __ _______
in reverse
Active Transport Refresher
ATP is hydrolyzed to ___ and __, and ____ ______ used to pump ___ from [___] to [____]
Remember that reactions are ___________.
ADP, Pi, free energy, H+, low, high, reversible
Reversal of this Transport
H+ moving across membrane from [____] to [___], release free energy that can be used to drive ___ _________
high, low, ATP synthesis
Where does the H+ gradient come from?
Some respiratory complexes can actively transport H+ across the inner membrane.
Use free energy released by transported electrons
Complexes I, III, and IV transport H+
No evidence that Complex II transports H+
Directional H+ transport, together with an impermeable inner membrane, means a substantial gradient can form
Remember that cells store energy in gradients
A H+ gradient is an electrochemical gradient
The total amount of stored energy results from the combined concentration and electrical differences across the membrane
_______ is a passive movement of water across a membrane
____________ is passive movement of ions (H+ in this case) across a membrane
More specifically, chemiosmosis refers to the process of ATP synthesis driven by the movement of ___ across a _________
osmosis, chemiosmosis, H+, membrane
The Chemiosmotic Model for ATP Synthesis
Exergonic transfer of electrons through the _____ ________drives active H+ transport across the ______ _________ and ___ __ ________
When the H+ cross the membrane back into the matrix, ____ ______ is released by spinning ___ ________
The energy is used to synthesize ___ from ___ and __
inner membrane, inner membrane, out of matrix, free energy, ATP synthase, ATP, ADP, Pi
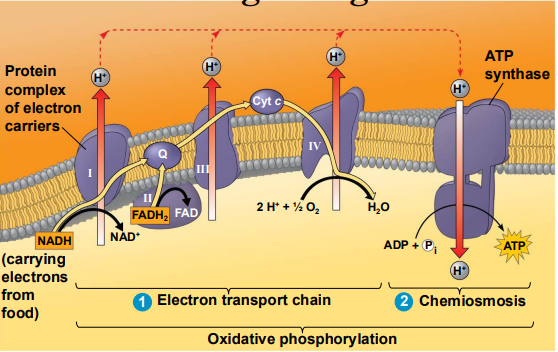
Putting It Together
For your reference
N/A
Oxidative Phosphorylation
The phosphorylation of ___ during _______ __________ is associated with the ______-__________ transport of electrons in the ____________.
This is called __________ ______________ to distinguish it from substrate-level phosphorylation
ADP, aerobic metabolism, oxygen dependent, mitochondria, oxidative phosphorylation
How much energy do the electrons have?
How much energy do electrons in NADH have?
How much energy do electrons in FADH2 have?
**Remember that Complex II does not transport __
enough energy to make between 2 and 3 ATPs
enough energy to make between 1 and 2 ATPs
H+
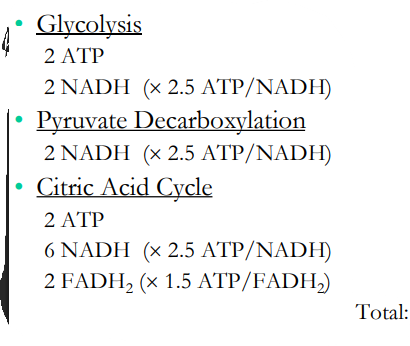
Final Cost Accounting
How many ATP produced after each step?
2
5
5
2
15
3
Total: 32
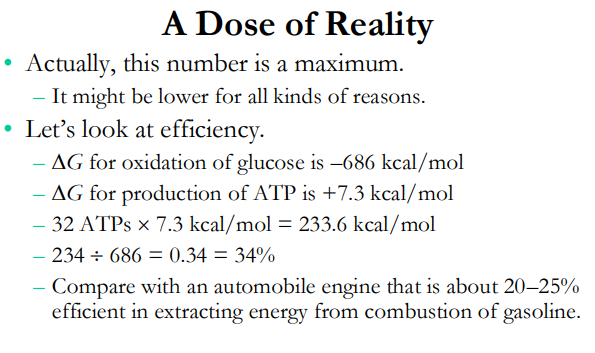
For your reference
N/A
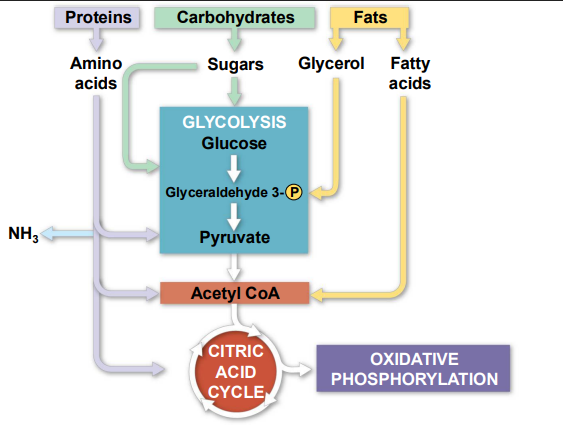
The Versatility of Cellular Respiration
Catabolic pathways funnel electrons from many kinds of _______ ________ into cellular respiration
Glycolysis accepts a wide range of ___________
________ must be digested to amino acids; amino groups can feed glycolysis or the citric acid cycle
organic molecules, carbohydrates, proteins
The Versatility of Cellular Respiration
Fats are digested to ________ (used in glycolysis) and fatty acids (used in generating _____ ___)
Fatty acids are broken down by ___ _________ and yield _____ ___
An oxidized gram of fat produces more than ______ as much ___ as an oxidized gram of ___________
glycerol, acetyl CoA, beta oxidation, acetyl CoA, twice, ATP, carbohydrate
For your reference
N/A
Regulation of Cellular Respiration via Feedback Mechanisms
______ _________ is the most common mechanism for metabolic control
If ATP concentration begins to drop, respiration ______ __; when there is plenty of ___, respiration _____ _______
Control of catabolism is based mainly on __________ ___ ________ __ _________ at strategic points in the catabolic pathway
feedback inhibition, speeds up, ATP, slows down, regulating the activity of enzymes