Session 3: Blood cells and haematopoiesis
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
111 Terms
What is haematopoiesis?
The process of producing mature blood cells from precursor cells
Where does haematopoiesis occur in the second trimester?
From the second trimester, haematopoiesis primarily occurs in the liver.
Where does haematopoiesis occur in the third trimester?
From the third trimester, haematopoiesis primarily occurs in the bone marrow
Where does haematopoiesis occur in adults?
Bone marrow of the sternum, pelvis, vertebrae, ribs and skull.
Where does haematopoiesis occur in the foetus?
The liver and spleen
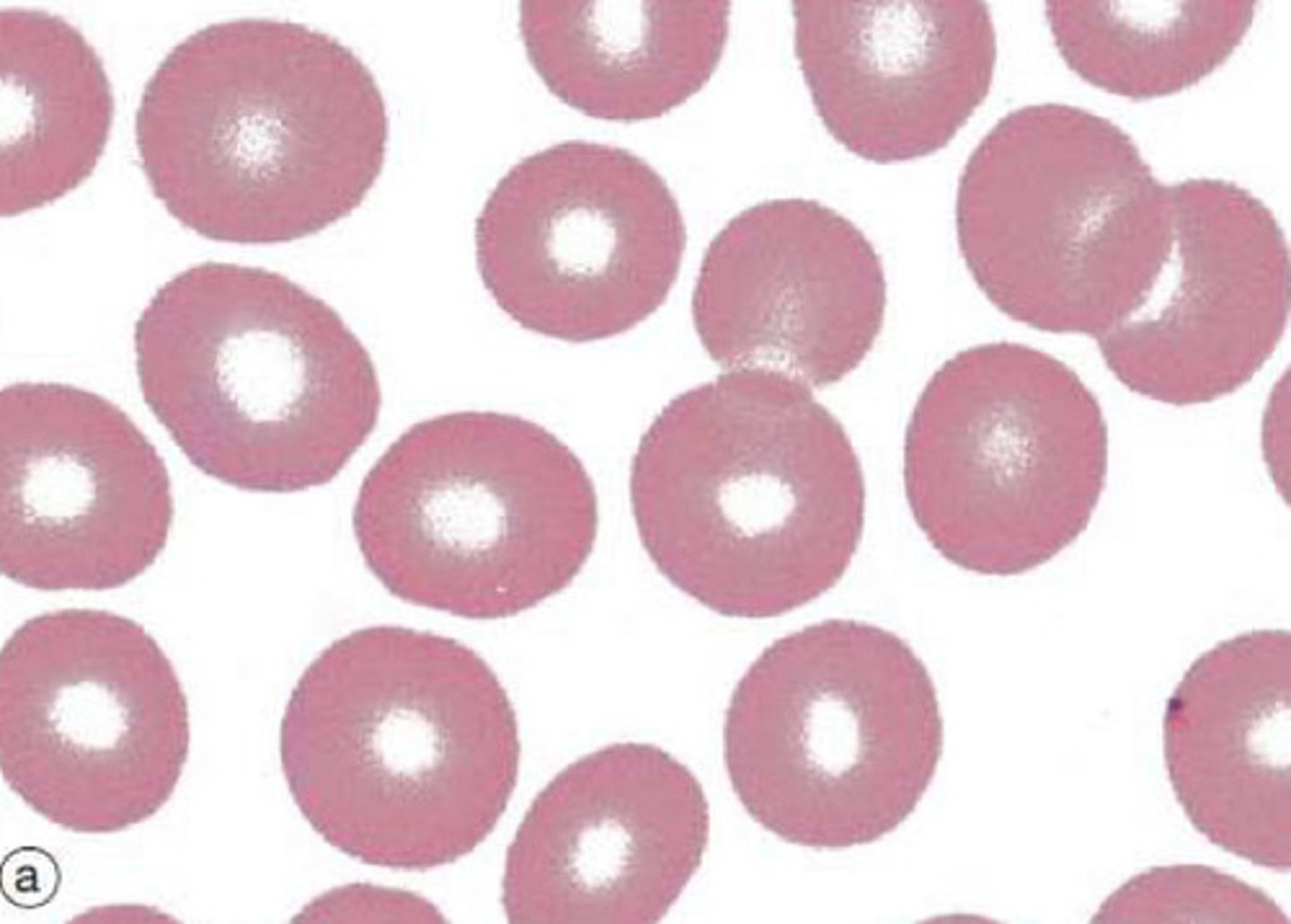
Describe the features of erythrocytes (RBCs)
Anucleate cells densely filled with haemoglobin - have no organelles
- Flexible, biconcave discs with a diameter of ~7.2uM (x2 diameter of narrowest capillaries)
- Large surface area for gaseous exchange for delivering oxygen to tissues and returning carbon dioxide to lungs
What is the progenitor of erythrocytes?
Myeloid stem cell
What is the process of production of erythrocytes?
Erythropoiesis
What controls erythropoiesis?
- An imbalance in homeostasis of blood oxygen levels and acidity of the blood acts as a stimulus = hypoxia due to decreased RBC count, decreased amount of haemoglobin, or decreased availability of oxygen
- This leads to reduced oxygen in the blood
- The kidney (and liver) then releases erythropoietin in response
- The erythropoietin stimulates red bone marrow to enhance erythropoiesis and increase the RBC count as a result
- The increased RBC count leads to increase of oxygen carrying capacity of the blood
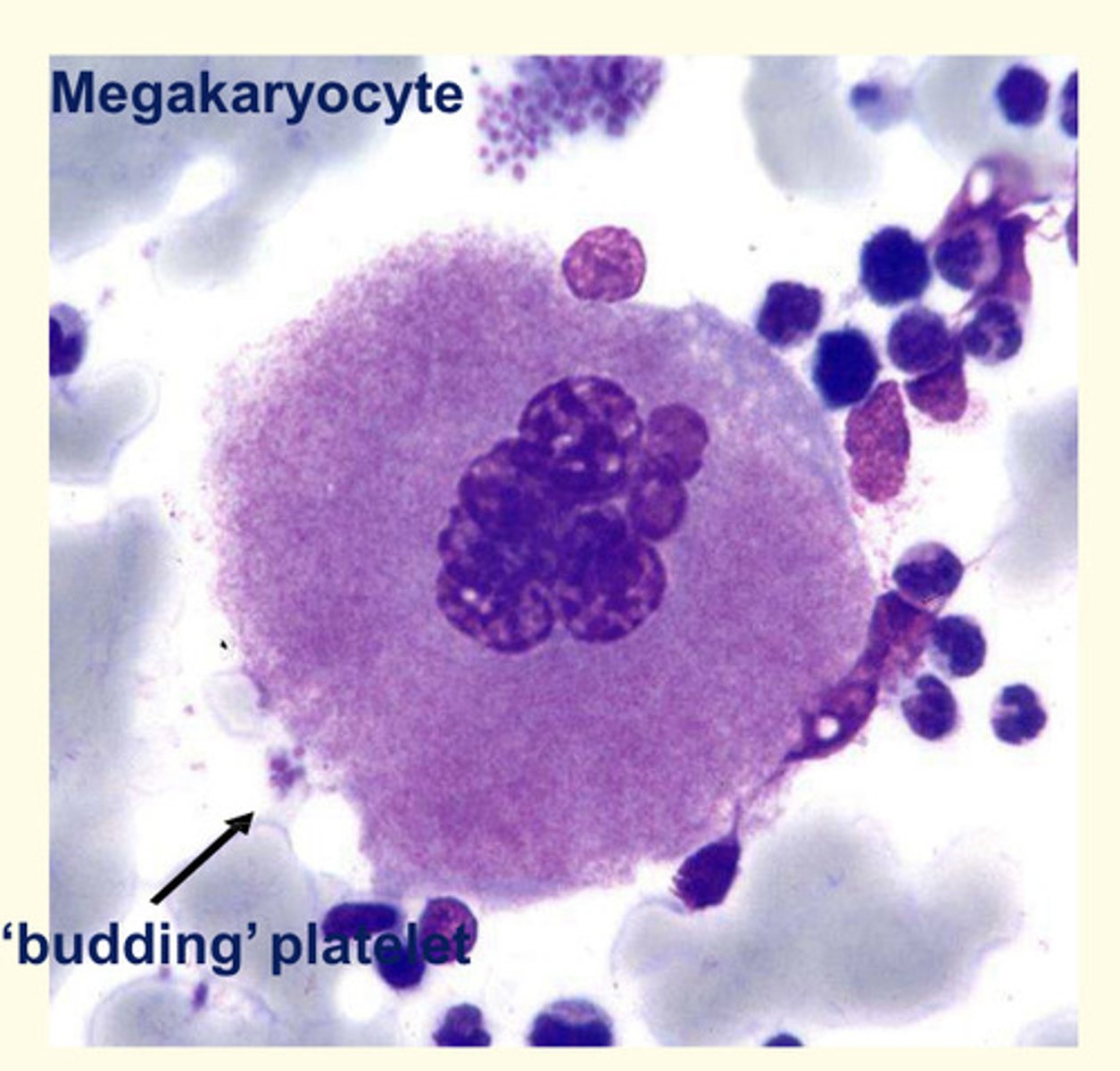
Describe the features of platelets?
- Platelets are anucleate 2-3uM cell fragments derived from bone marrow megakaryocytes.
- They contain a range of factors which are important for blood clotting and vessel wall repair
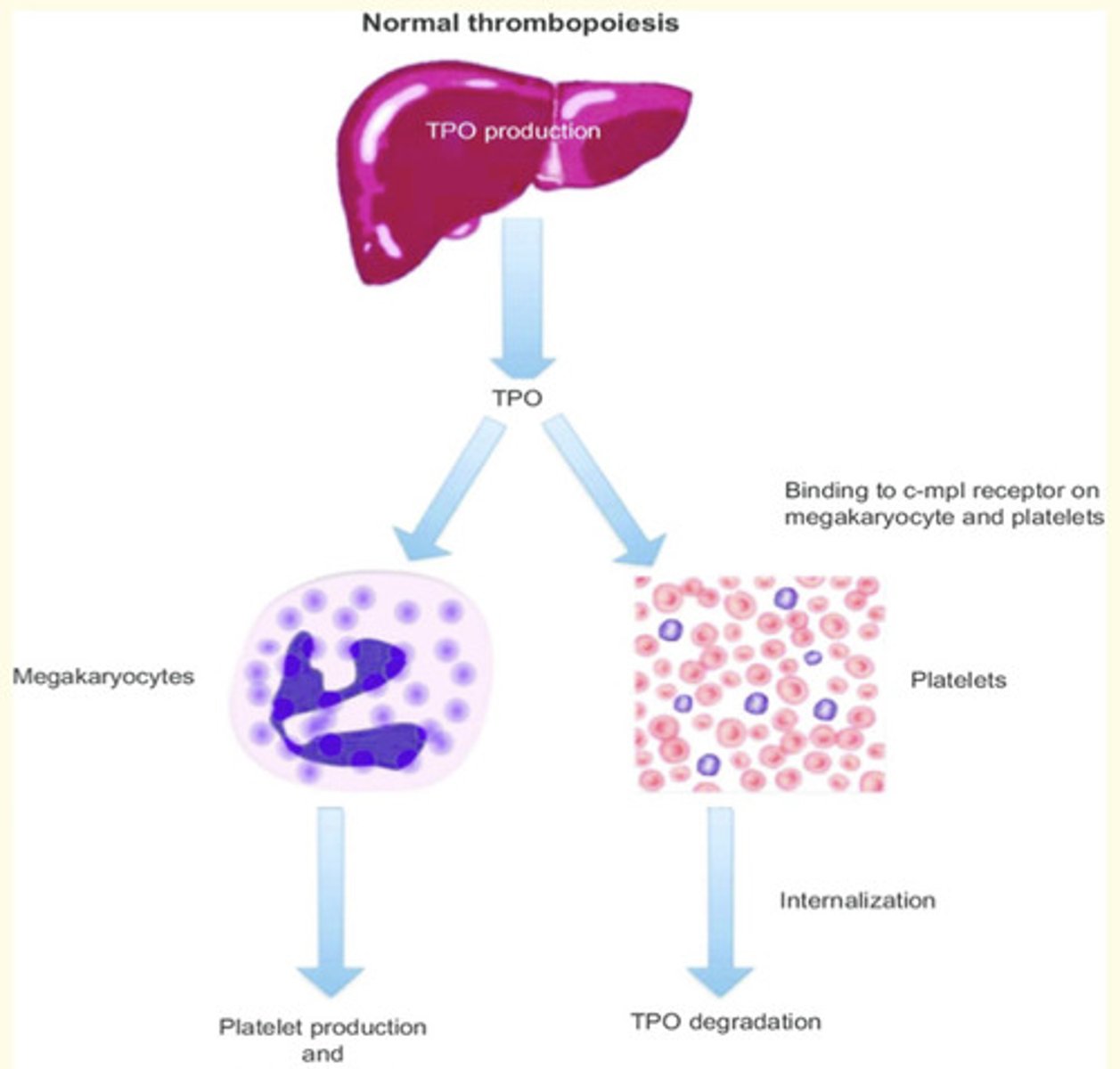
What is thrombopoiesis?
Thrombopoiesis is the formation of thrombocytes in the bone marrow
What is the main regulator of thrombopoiesis?
Thrombopoietin hormone (TPO)
Where is thrombopoietin produced?
By the liver and kidneys
How does thrombopoietin (TPO) work?
TPO acts in the bone marrow to stimulate megakaryocytes to increase in size = by undergoing DNA replication without dividing
- Platelets 'bud off' or ligate from enlarged cells
- TPO can bind to platelets, where it is destroyed = this reduces the bioavailability of the hormone as the platelet numbers rise.
What is innate immunity?
An immediate, yet non-specific and transient response to infection
What is adaptive immunity?
- Humoral responses involve secretion of immunoglobulins (antibodies) by B cells
- Cell-mediated responses involve the killing of infected cells by T cells
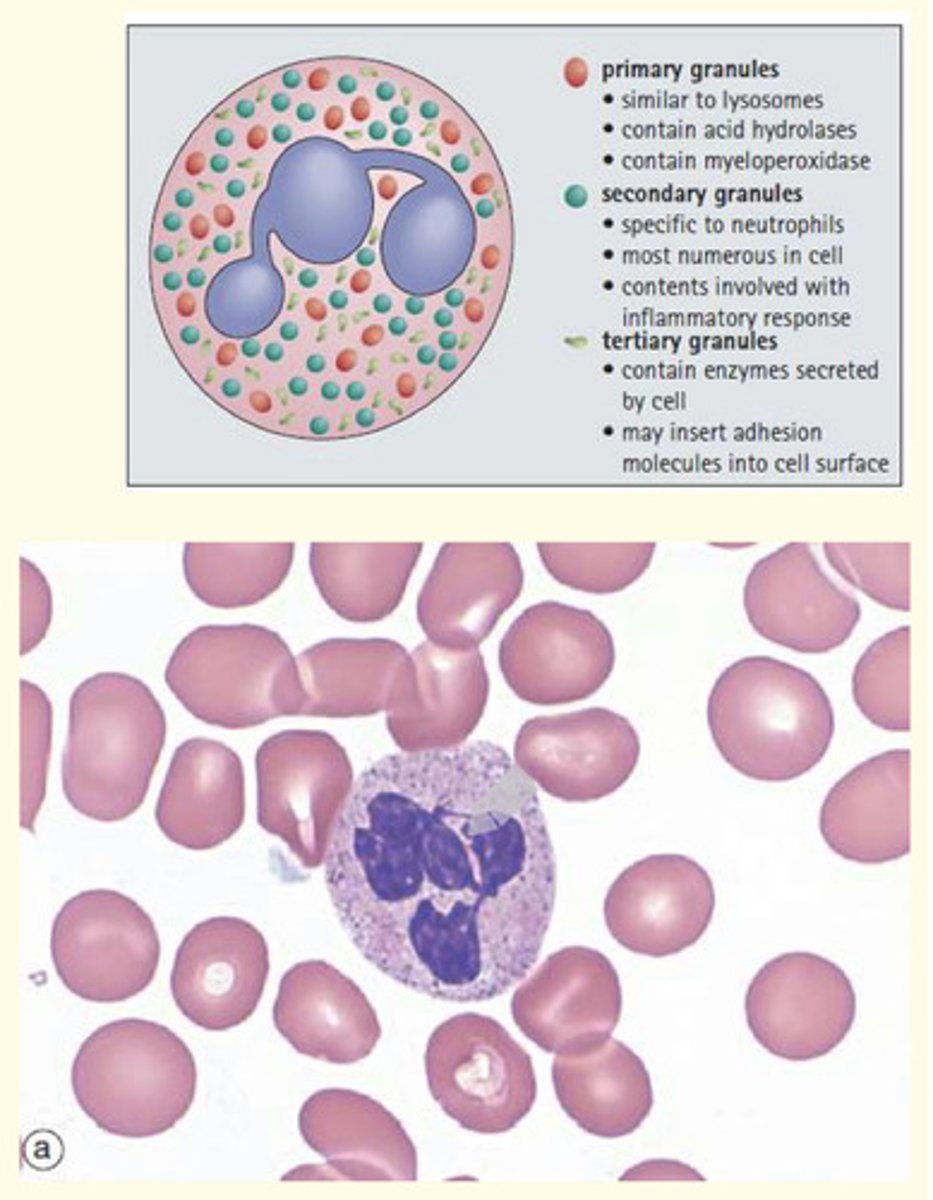
What are neutrophils?
Most common circulating leukocyte (40-70% of WBC).
Lifespan of 1-4 days
Lobed nucleus (polymorphonuclear cells)
Recruited to sites of infection to phagocytose pathogens
Granules inside them contain proteases, antibacterial peptides and oxidising agents
12-15 uM diameter
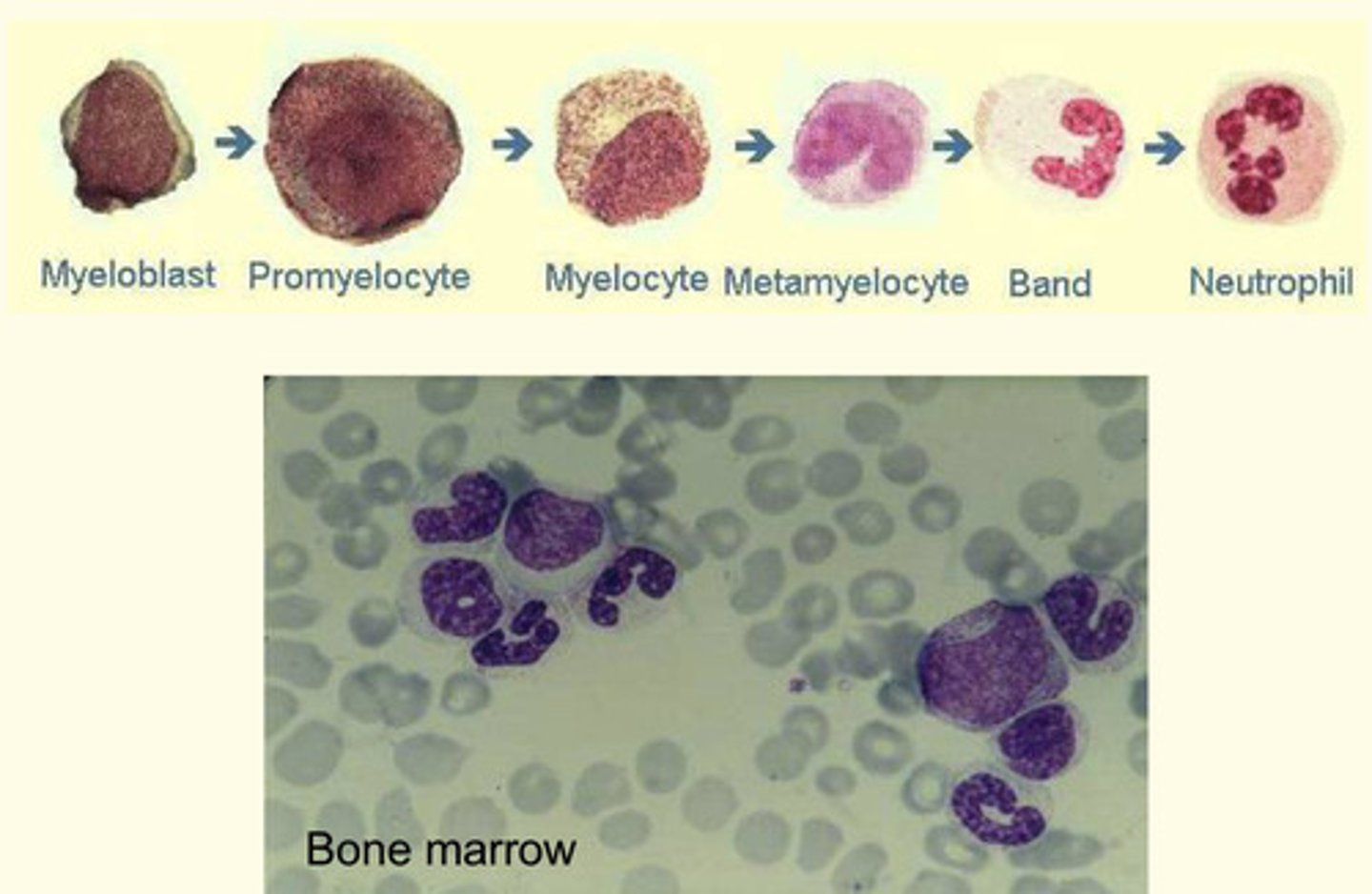
Where do neutrophils develop?
Neutrophils develop in the bone marrow
What are eosinophils?
Account for 1-6% of circulating leukocytes
Lifespan of 6-9 days
Red granules revealed with Wright's stain (appear eosinophilia with H&E stain)
Functions: combatting HELMINTH infection, mediating hypersensitivity reactions (allergies) and phagocytosis antigen-antibody complexes
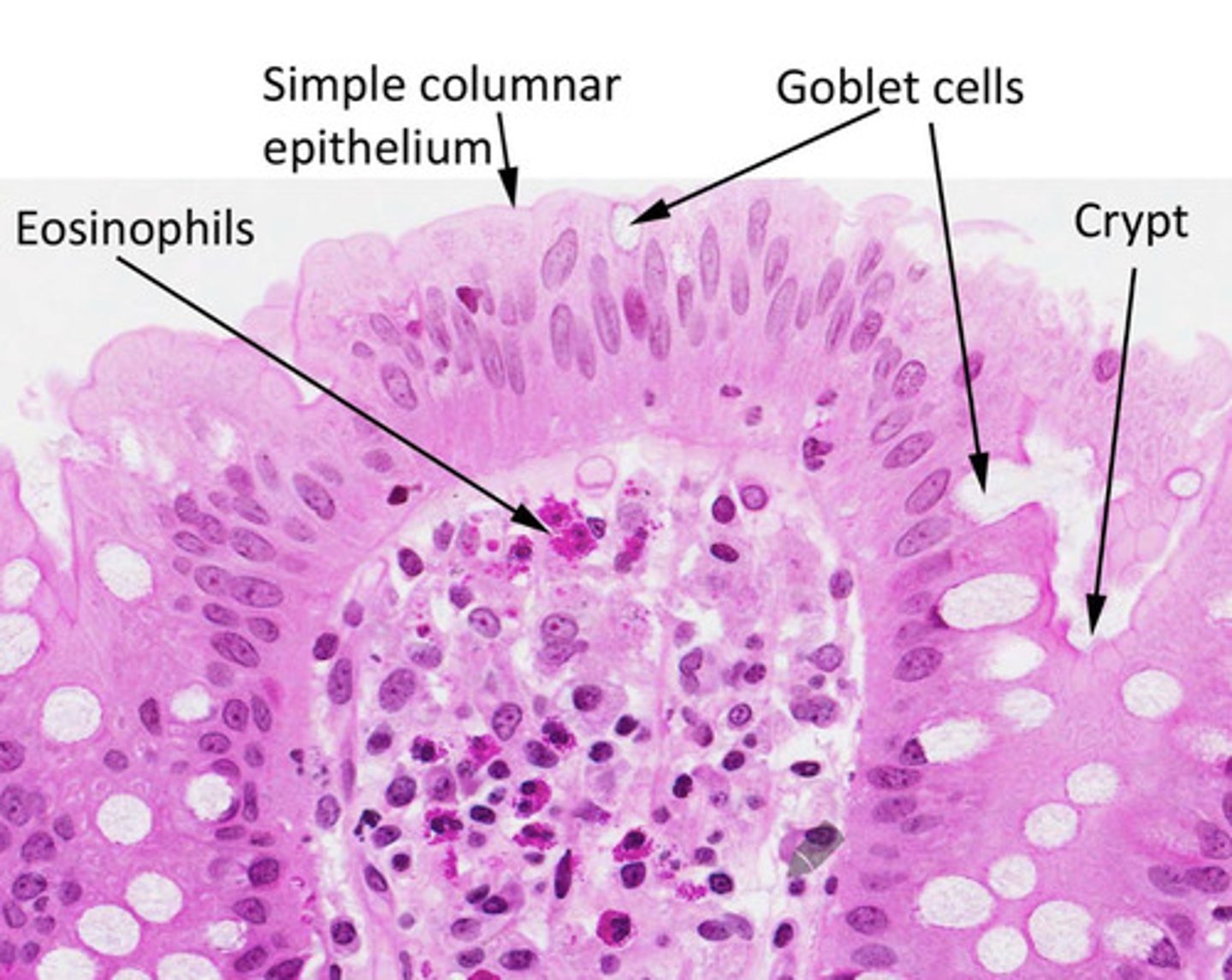
In what tissue are abundant eosinophils found?
Eosinophils are abundant in the connective tissue of the intestinal lining and in the lungs of asthma patients.
What are basophils?
Least-common WBC (0.2-1% of circulating leukocytes)
Half-life 2.5 days
Purple-staining of basophilic granules
Cytoplasm contain histamine, heparin and pro-inflammatory cytokines
Function: importance in type 1 hypersensitivity reactions (e.g. re-exposure to allergens)
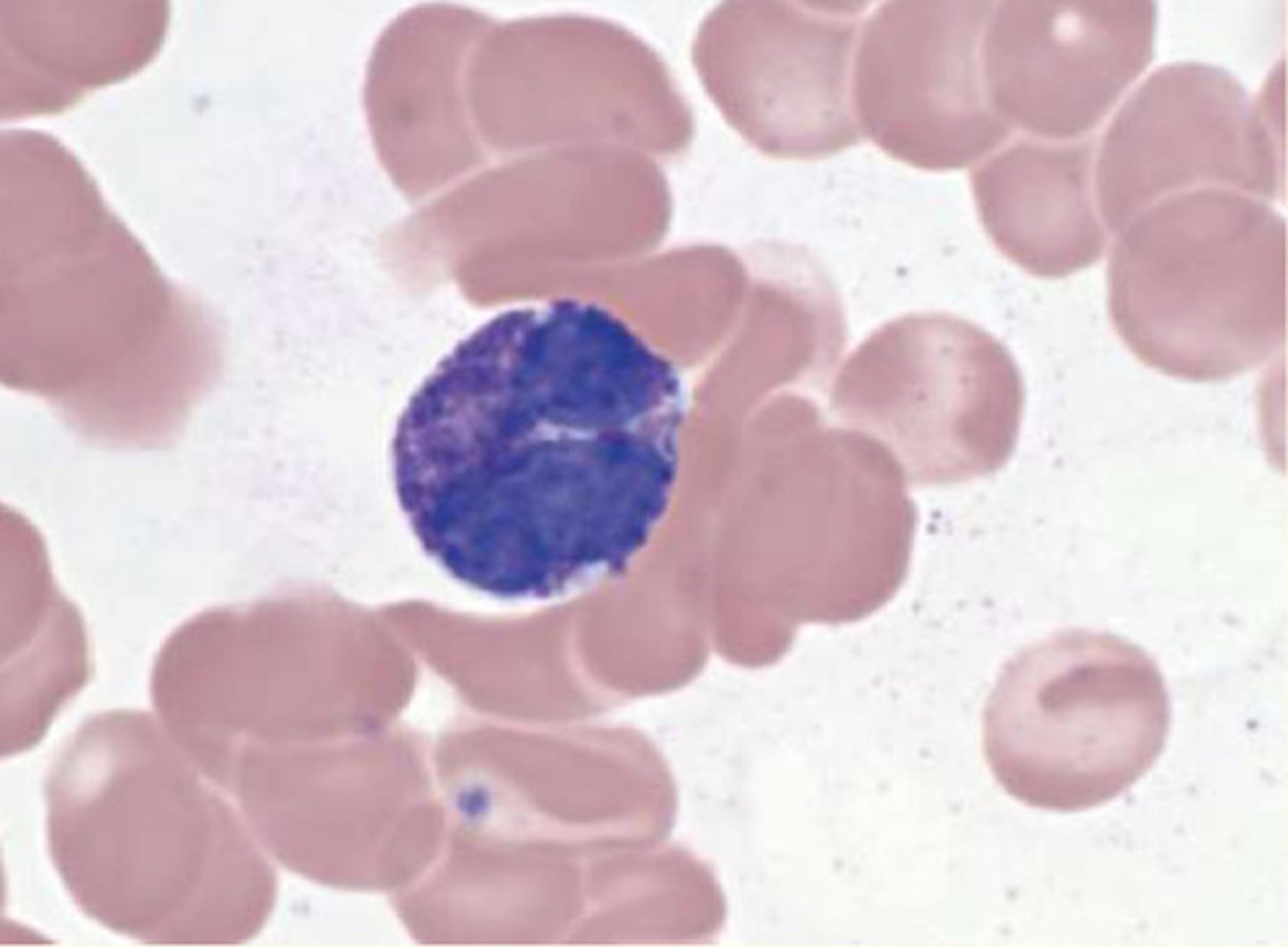
What are monocytes?
Account for 2-10% of total WBC
Lifespan varies (days)
Phagocytic and pinocytic
Monocytes are 'agranulocytes' that leave circulatory system by diapedesis and mature into macrophages in tissues
15-20uM diameter
Large, kidney-shaped nucleus
Fine cytoplasmic granules contain lysosomes
Function: respond to inflammation and act as antigen-presenting cells (APCs)
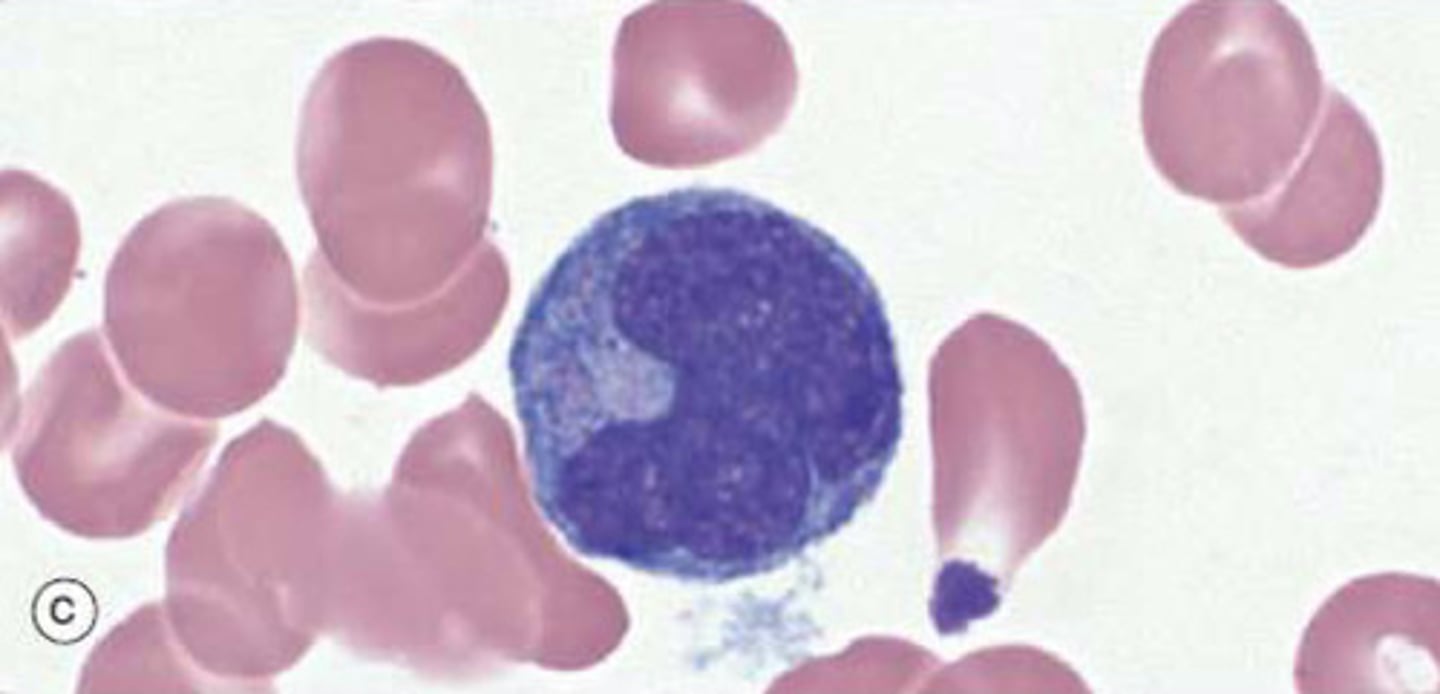
What are macrophages?
Lifespan of several years
Derived from circulating monocytes which migrate to loose connective tissue
Function: respond to local inflammation and phagocytic (degrade foreign organisms/cell debris)
Professional antigen presenting cells (APCs) which present foreign materials to T-lymphocytes
What is adaptive (or acquired) immunity is divided into two systems?
1. Humoral immunity: secretion of immunoglobulins by B lymphocytes into extracellular fluids (humours)
2. Cell-mediated immunity: T lymphocyte-mediated destruction of infected cells (via cytotoxic lysozymes).
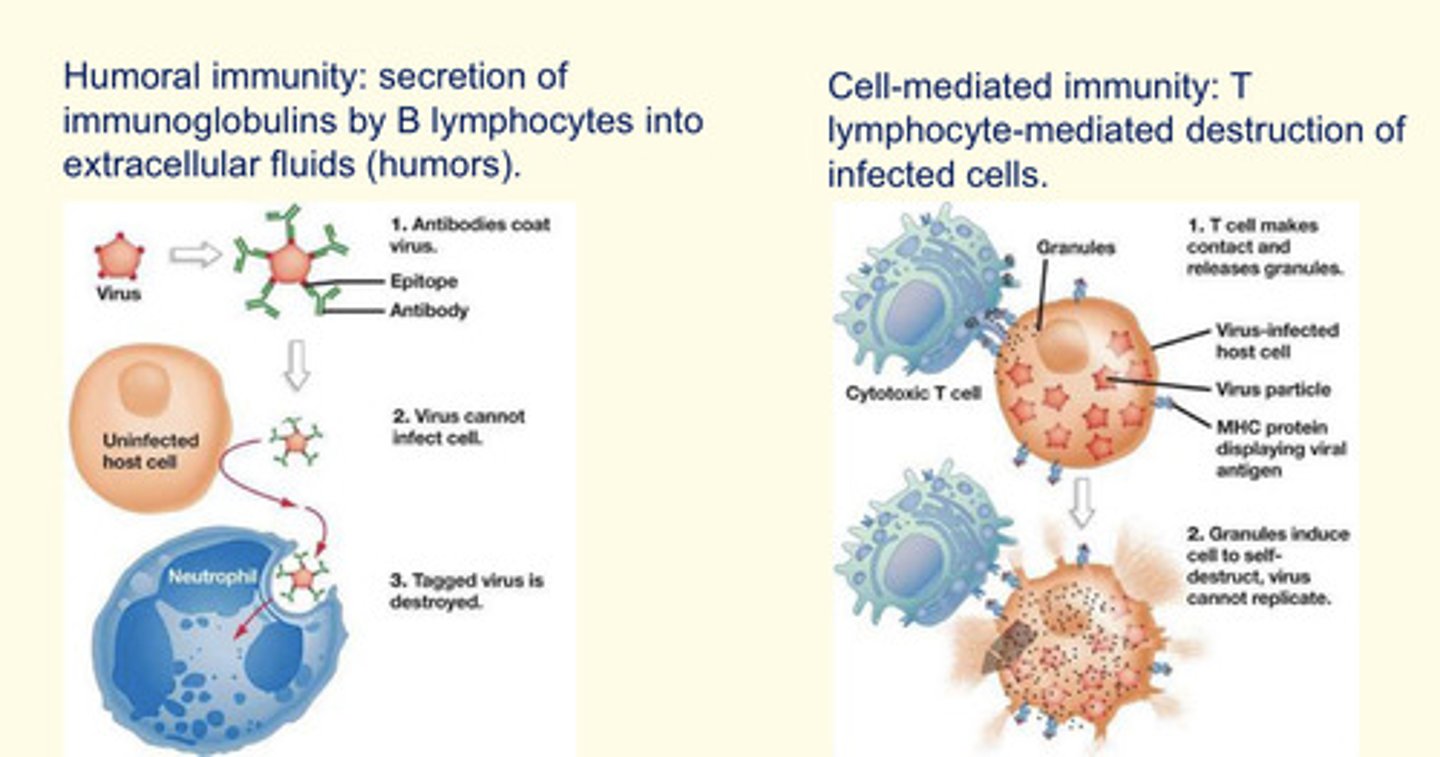
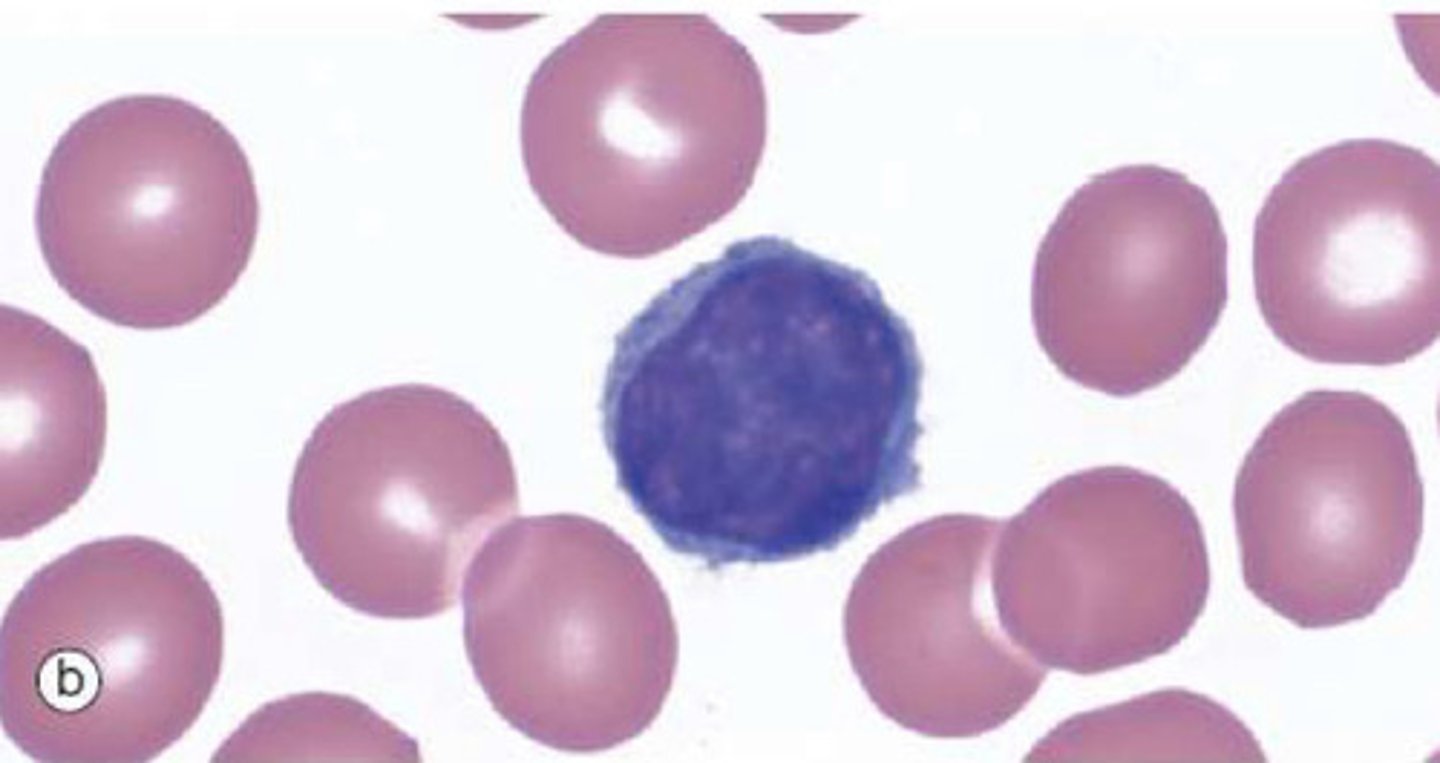
What are lymphocytes?
White blood cells which constitute 20-50% of circulating leukocytes.
5-20uM in diameter
Appear spherical in histological preparations with thin strip of cytoplasm surrounding large but regularly shaped nucleus
B and T lymphocytes
How do erythrocytes survive if they have no organelles (mitochondria) to produce ATP via oxidative phosphorylation?
They get their energy from glycolysis in the cytoplasm (make ATP using glucose - does not involve mitochondria).
What is the function of B lymphocytes?
to shuffle DNA encoding their immunoglobulins to create a range of antibodies able to recognise a range of antigens.
What does a B cell do if presented to a foreign antigen it recognises?
The B cell will proliferate under the control of T-helper cells - to form a population of plasma cells that will produce antibodies specific for that antigen.
What is the name given to B lymphocytes which are able to expand again following re-exposure to the same antigen?
Long-lived memory B lymphocytes
What are T lymphocytes?
Lymphocytes that originate in the bone marrow but mature in the thymus (or spleen in adults). They undergo rearrangement of their T cell receptor genes. T lymphocytes are made up of CD4+ (T helper) cells and CD8+ (T cytotoxic) cells
What is the function of CD4+ (T helper) cells
CD4+ T-helper cells induce proliferation and differentiation of T and B cells and activate macrophages
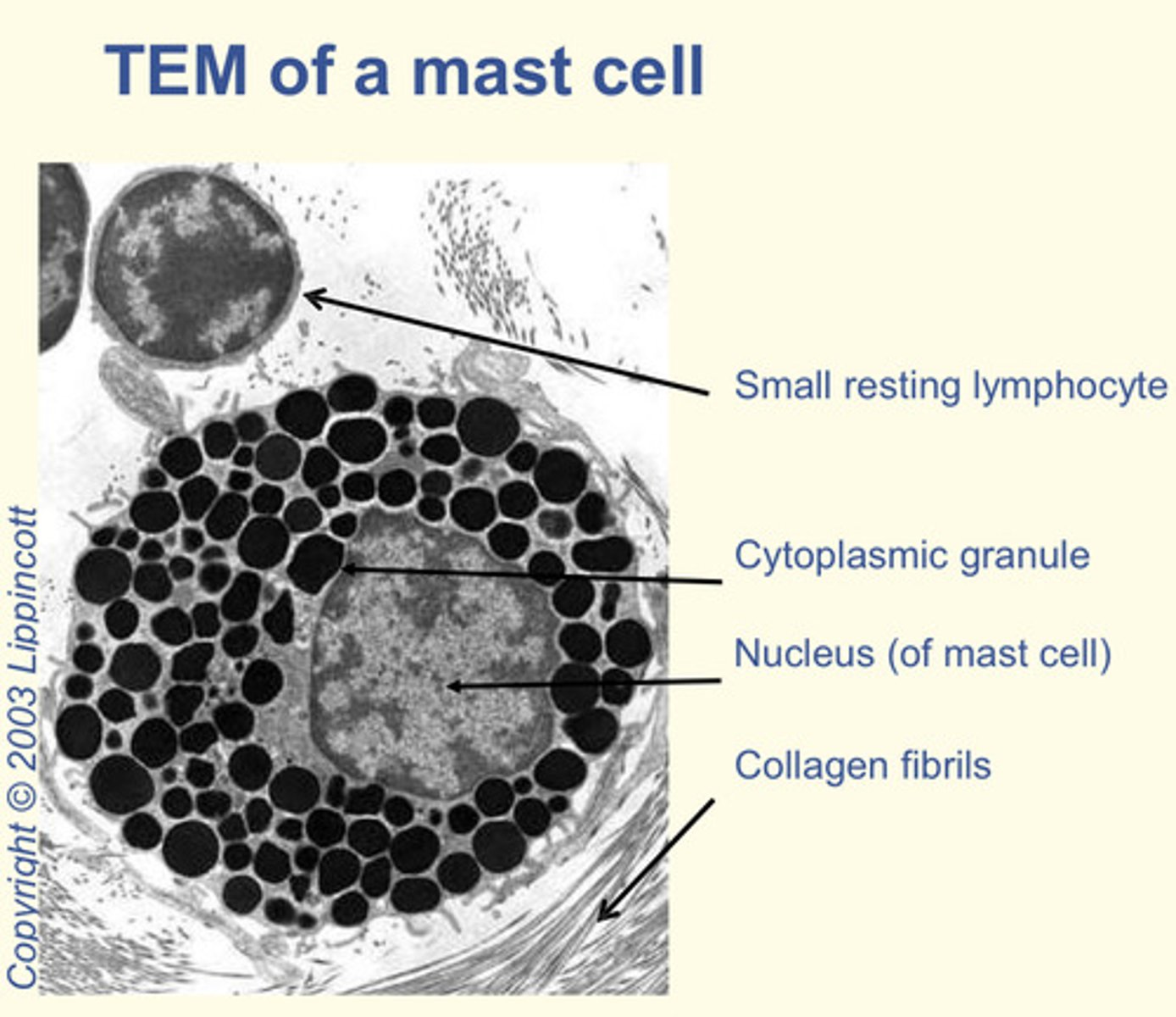
What are mast cells?
They appear like basophils but are derived from a DIFFERENT precursor
Components of the innate immune system but also play role in allergy
Granules contain: heparin (anticoagulant), histamine (increase blood vessel wall permeability) and cytokines (to attract eosinophils and neutrophils)
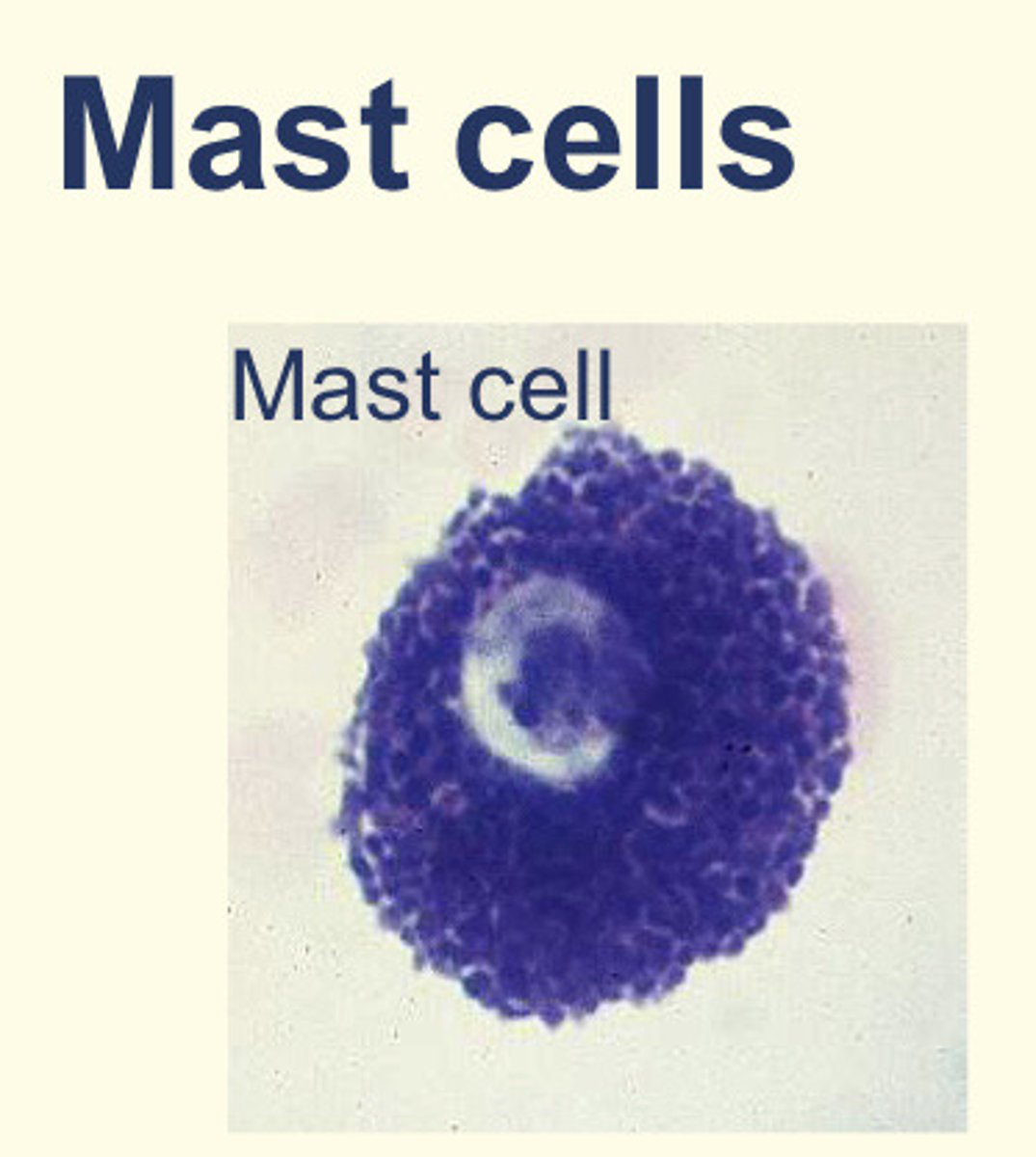
Where can mast cells be found?
Connective tissue
What is the function of CD8+ (T cytotoxic) cells
induce apoptosis in virally-infected cells by creating holes in the plasma membrane with perforin
injecting granzymes which degrade the cell
What is the name given to the pathological response resulting from mast cell hypersensitivity?
Type 1 hypersensitivity.
This is stimulated by allergens.
Responses can be localised (allergy) or systemic (anaphylaxis).
Describe the process of type 1 hypersensitivity resulting from mast cell hypersensitivity?
Following initial exposure, mast cells become coated with immunoglobulin E (IgE) molecules that specifically bind allergens
When an allergen cross-links these surface-bound IgE molecules, the contents of the granules are rapidly released from the cell.
Describe structure/function of RBC (erythrocytes)
Structure: anucleate cells, specialised for mechanical flexibility, 120-day lifespan, contain concentrated haemoglobin
Function: oxygen and carbon dioxide carriage
Describe structure/function of neutrophils
Structure: end cells; cannot re-enter blood.
Numerous granules with proinflammatory and antibacterial products.
Phagocytose and kill bacteria.
Function: inflammation and defence against bacteria
Describe structure/function of eosinophils
Structure: end cells; cannot re-enter blood. Numerous granules with proinflammatory and anti-parasite products.
Function: Inflammation, allergy defence and defence against parasites
Describe structure/function of basophils
Structure: end cells; cannot re-enter the blood. Numerous granules with proinflammatory products.
Function: Inflammation, allergy and defence against parasites.
Describe structure/function of monocytes
Structure: can mature into macrophages - including becoming long-term tissue macrophages. Ingest organisms (phagocytosis) and debris. Major cytokine producers
Function: Inflammation and defence against infections
Describe structure/function of lymphocytes
Structure: after formation can proliferate in tissues and lymph nodes and recirculate through the blood
Function: adaptive immune system
Describe structure/function of platelets (thrombocytes)
Structure: cell fragments produced by fragmentation of megakaryocyte cytoplasm - major source of growth factors at sites of injury
Function: blood clotting (haemostasis)
Which one of the following most accurately describes the functions of mast cells?
A) Phagocytosis
B) Immunoglobulin production
C) Killing of virally-infected cells
D) Local inflammation, innate immunity, tissue repair
E) Antigen presentation
D) Local inflammation, innate immunity, tissue repair
What is the function of 'chemotactic factors' produced by mast cells?
Recruiting immune cells, particularly eosinophils and neutrophils
What is the function of 'histamine' produced by mast cells?
Increase vascular permeability
What is the function of 'cytokines' produced by mast cells?
Modulators of immune cell activity
What is the function of 'heparin' produced by mast cells?
Anti-coagulant
From which immune cell type do macrophages develop?
Monocytes
Where do macrophages mature?
In organs such as the skin and liver
Arrange these blood components according to life span length with 1 being the shortest and 4 being the longest.
RBCs, Neutrophils, Platelets, Memory B cells
1: Neutrophils (shortest life-span)
2: Platelets
3: RBCs
4: Memory B cells (longest life-span)
What are the names of the cells which detect reduced pO2?
Peritubular cells of the kidney
What is the name of the hormone which is released by peritubular cells of the kidney to promote maturation of erythrocyte precursors?
Erythropoietin (EPO)
Where are erythrocyte precursors found?
Bone marrow
What is the name of the hormone which regulates platelet homeostasis?
Thrombopoietin
The hormone _______ stimulates ______ cells in the bone marrow to enlarge.
The hormone thrombopoietin stimulates megakaryocyte cells in the bone marrow to enlarge.
Thrombopoietin is produced constitutively by the ______ and ______.
Thrombopoietin is produced constitutively by the kidney and liver.
Match the circulating leukocyte to the differential count.
Monocytes
Neutrophils
Lymphocytes
Basophils
Eosinophils
Basophils: 0-0.75% of WBCs
Eosinophils: 1-3% of WBCs
Monocytes: 3-7% of WBCs
Lymphocytes: 25-33% of circulating WBCs
Neutrophils: 57-67% of WBCs
Which cell provides defence against HELMINTH infection?
Eosinophils
Which cell provides phagocytosis against bacteria in particular
Neutrophils
Which cells provide adaptive immunity?
T and B lymphocytes
What component of blood is important for clotting?
Platelets
What cells are important for oxygen transport?
Red blood cells
Which cells are mildly phagocytosis APCs that differentiate into macrophages in tissues?
Monocytes
Which cells are important for allergic reactions?
Basophils
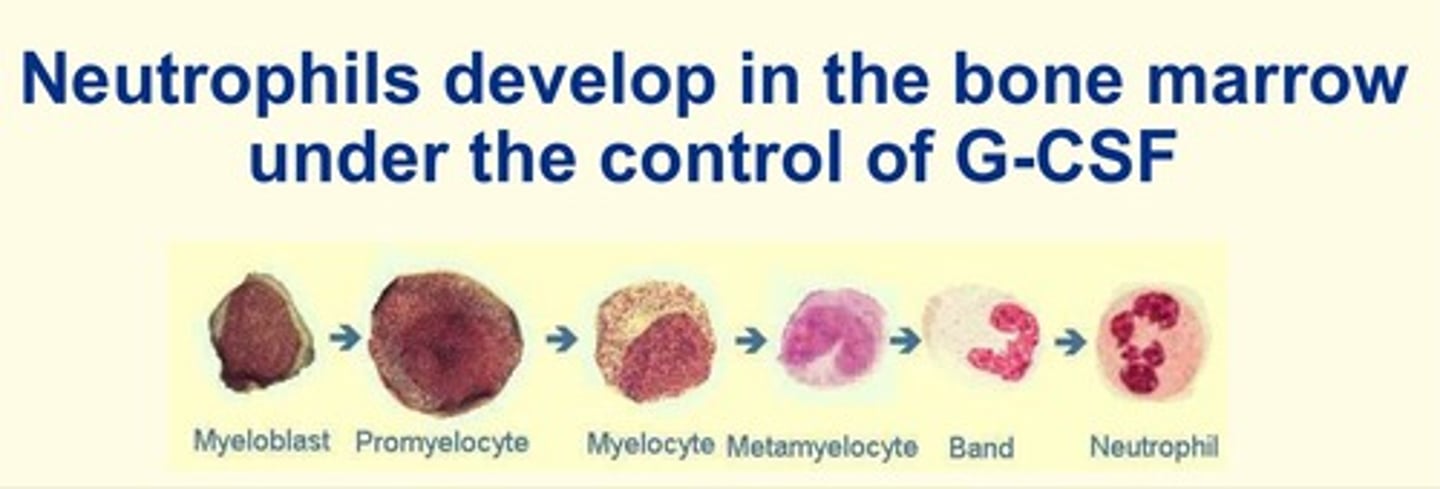
Order the following stages of neutrophil maturation, with the earliest being 1 and the latest being 6
Band cell
Myelocyte
Neutrophil
Myeloblast
Promyelocyte
Metamyelocyte
Myeloblast (earliest)
Promyelocyte
Myelocyte
Metamyelocyte
Band cell
Neutrophil (latest)
Where are dust cells found?
Lung
Where are Langerhans cells found?
Epidermis
Where are sinusoidal macrophages found?
Spleen
Where are Kupffer cells found?
Liver
Which lymphocyte kills "stressed cells" in an MHC-independent fashion?
Natural killer (NK) cells
Which lymphocyte are 'lymphoid progenitor cells'?
Lymphoblastic
Which lymphocyte produces immunoglobulins?
Plasma cells
Which lymphocyte suppresses immune functions?
CD4+ CD25+ (regulatory) T-cells
Which lymphocyte regulates immune functions e.g., in activating B cells?
CD4+ (helper) T-cells
Which lymphocyte kills virally-infected cells in an MHC-dependent fashion?
CD8+ cytotoxic T-cells
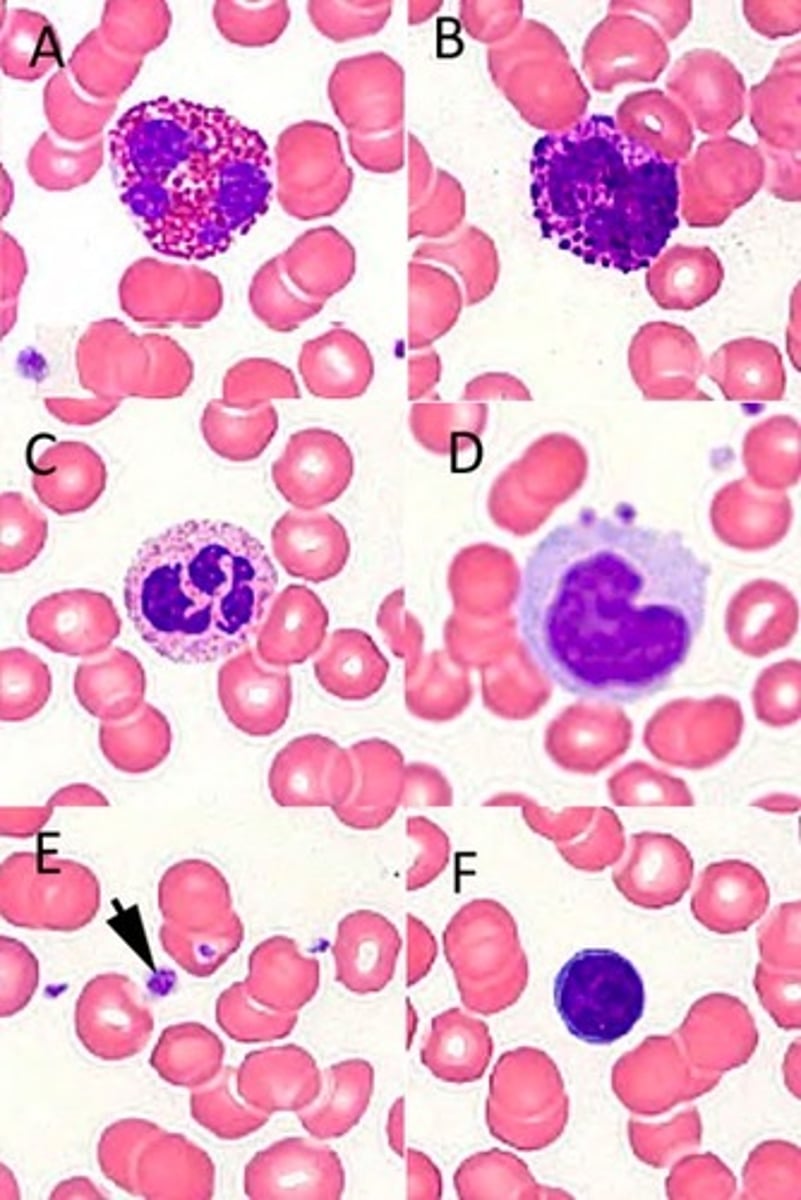
Identify the blood cells/components in the following high-power photomicrograph of a Giemsa-stained blood smear?
A: Eosinophil
B: Basophil
C: Neutrophil
D: Monocyte
E: Platelet
F: Lymphocyte
What stain is typically used when investigating WBC count in a bone marrow biopsy to assist in the diagnosis of a condition like chronic myeloid leukaemia (CML)?
Wright's stain
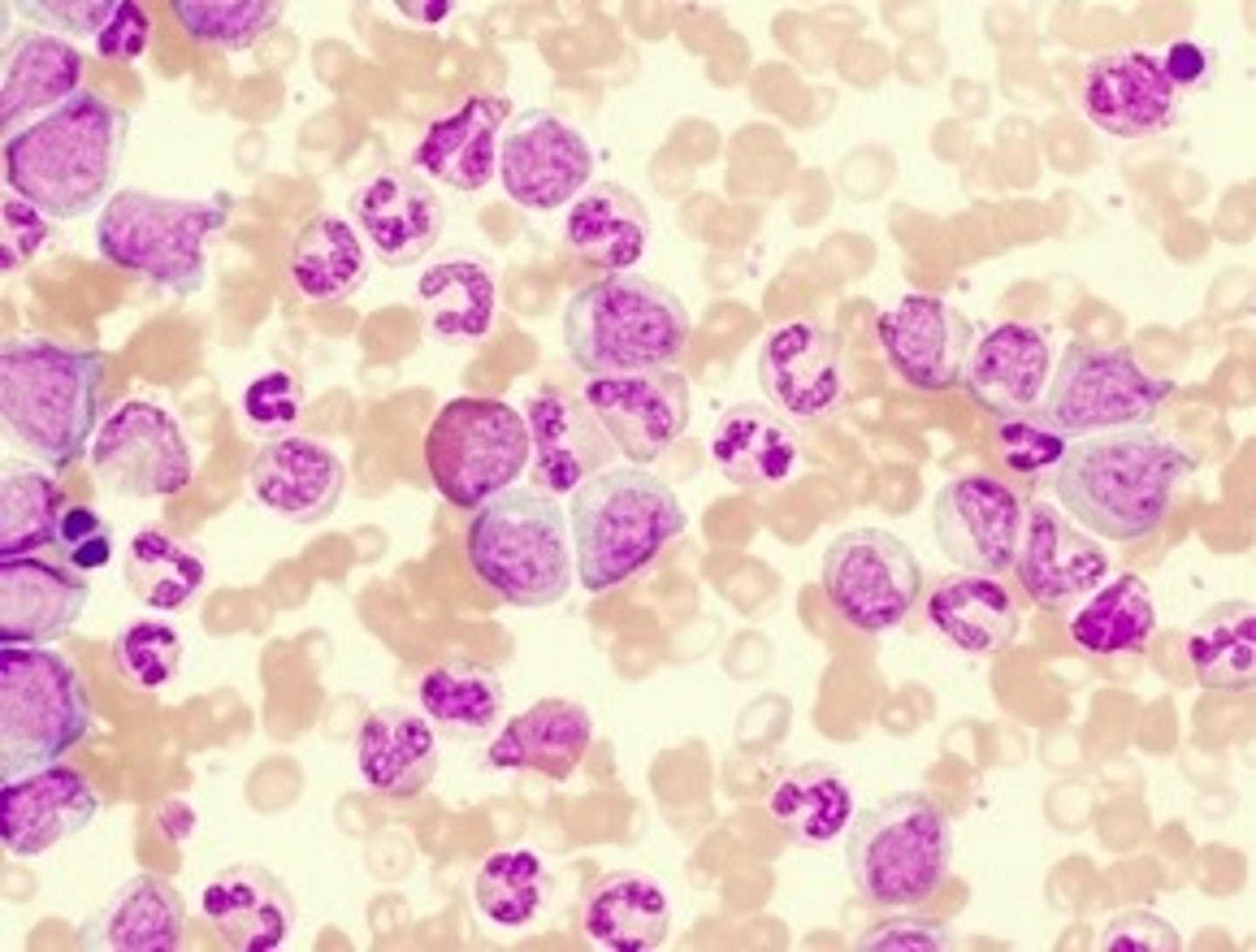
Which cell type would you NOT expect to see in a normal peripheral blood film?
A) Band cells
B) Lymphoblasts
C) Reticulocytes
D) Megakaryocytes
E) Macrophages
A) Band cells
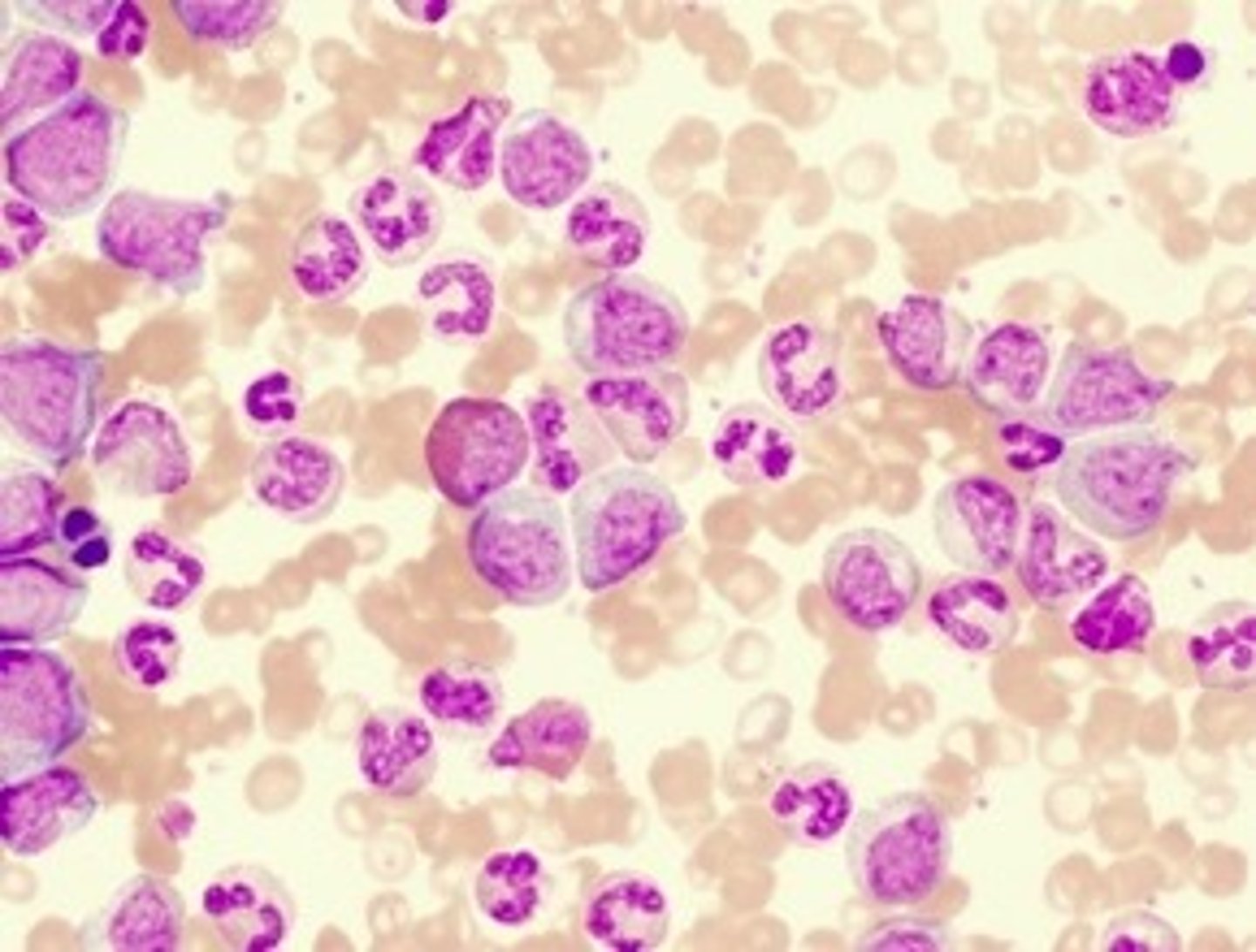
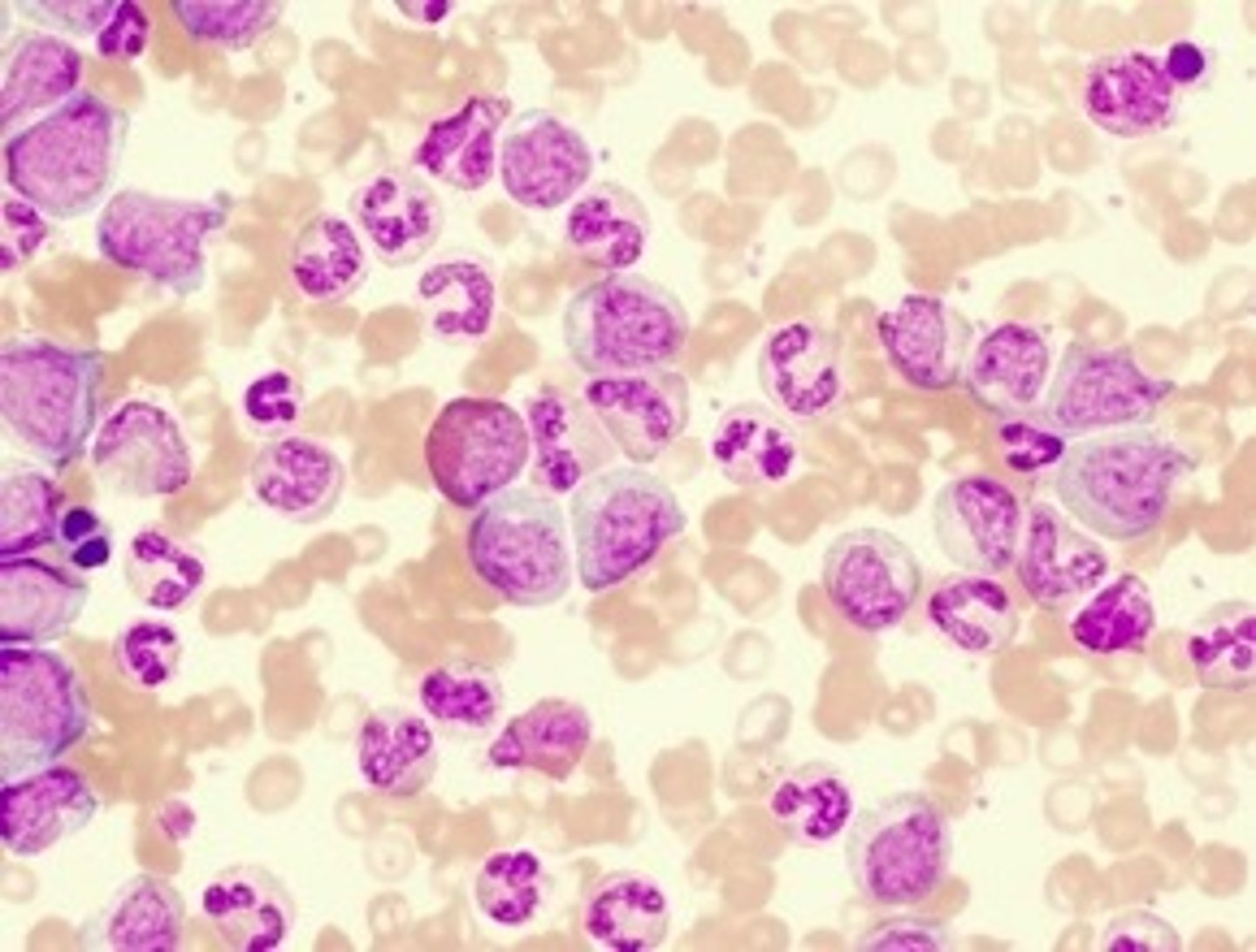
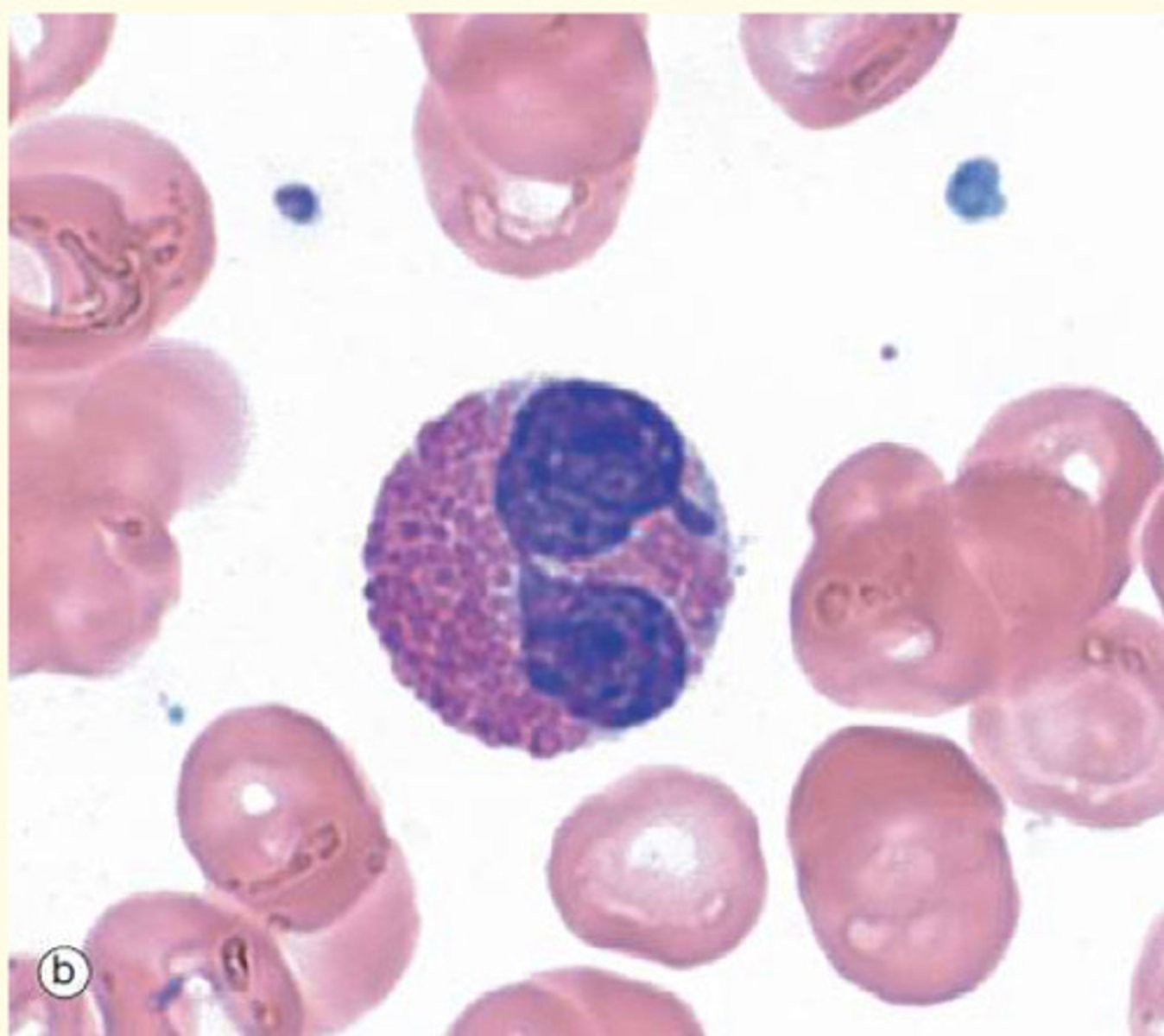
Where would the band cells visible in this blood film below normally be found?
In the bone marrow
From which site would bone marrow samples most likely be collected?
A) Rib
B) Vertebra
C) Head of the femur
D) Superior iliac crest
E) Sternum
D) Superior iliac crest
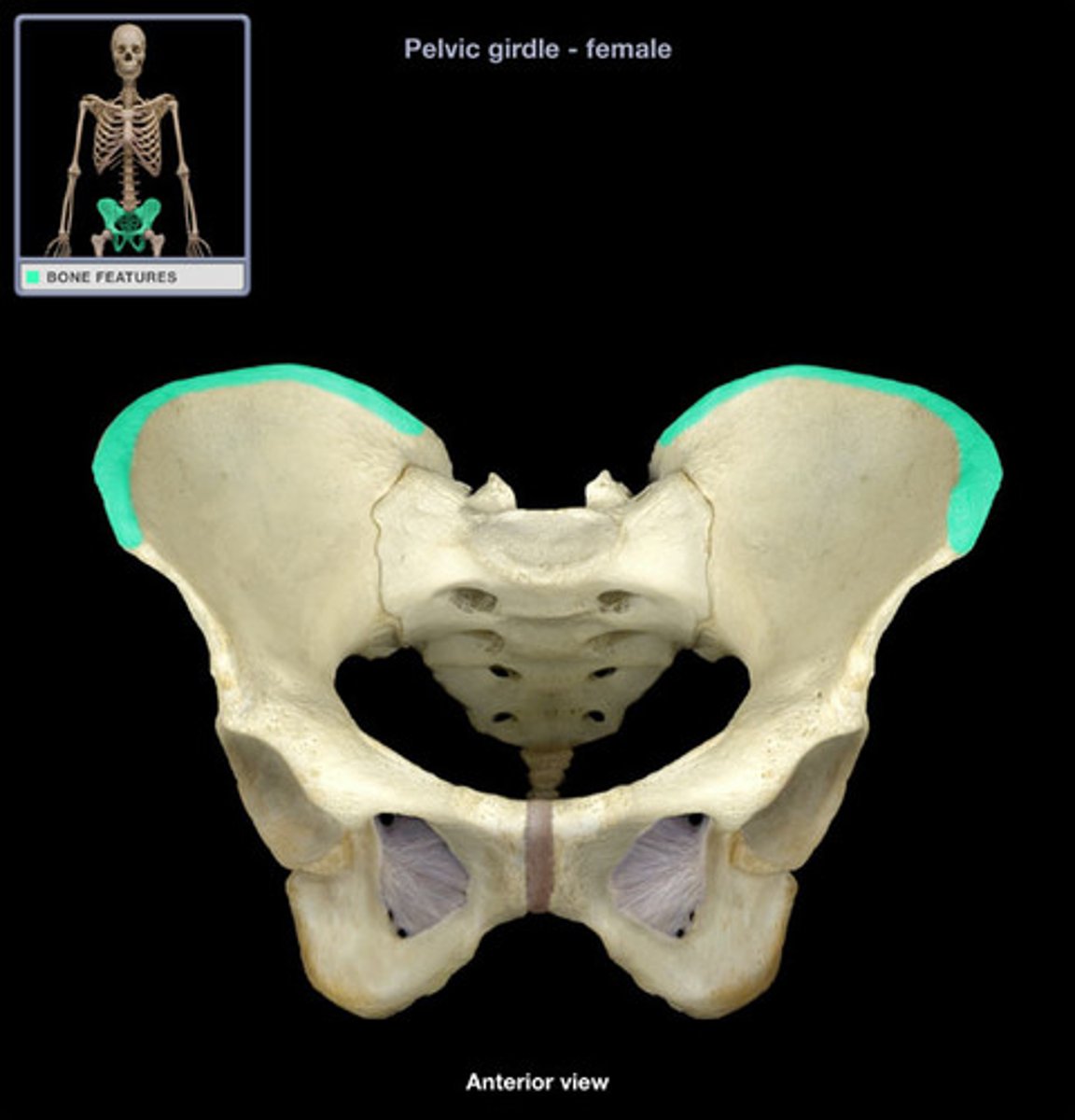
By which method would a bone marrow sample most likely be collected?
Trephine biopsy
What class of drugs is used to treat Chronic Myeloid Leukaemia (CML)?
Tyrosine kinase inhibitors
Jaundice results from the deposition in the skin of which product of haemoglobin catabolism?
Bilirubin
What cells in the reticuloendothelial system is responsible for destroying damaged RBCs?
Macrophages
In which organ are platelets stored?
Spleen
Activation of WHICH receptors on the surface of basophils (and mast cells) leads to degranulation, and is important in hypersensitivity reactions?
IgE
Which tissue-resident immune cell shares many features with basophils?
Mast cells
Which T-cell population is depleted in HIV infection?
Helper-T cells (CD4+)
Which immune structure monitors blood, as opposed to lymph, for infectious agents?
Spleen
In which of the following bones of adults does relatively LITTLE haematopoiesis take place in adults?
A) Pelvis
B) Femur
C) Scapula
D) Vertebrae
E) Skull
F) Ribs
B) Femur
Which one of the following interleukins is primarily involved in the development of myeloid lineages?
A) IL-2
B) IL-3
C) IL-6
D) IL-7
E) IL-12
B) IL-3
Which one of the following metabolic processes does NOT occur in RBCs?
A) Pentose phosphate pathway
B) Glycolysis
C) TCA cycle
D) Lactic acid fermentation
C) TCA cycle
Why is sickle cell anaemia not manifested in utero?
Beta chains progressively replace gamma chains from birth onwards
In sickle cell anaemia a ______ valine residue replaces a ______ glutamic acid at position 6 of the beta-globin gene as a consequence of a point mutation. Under hypoxic conditions, the altered haemoglobin ______, leading to a rigid, sickle-shaped cells that block microvasculature.
In sickle cel anaemia a non-polar/hydrophobic valine residue replaces a negatively-charged glutamic acid at position 6 of the beta-glob in gene as a consequence of a point mutation. Under hypoxic conditions, the altered haemoglobin polymerises, leading to a rigid, sickle-shaped cells that block microvasculature.
Which organ most commonly sustains damage due to the accumulation of damaged RBCs in an anaemic state?
Spleen
What is stercobilin?
breakdown product of conjugated bilirubin in the gut that gives stool brown color
What is bilirubin?
a orange-yellow pigment formed in the liver by the breakdown of hemoglobin and excreted in bile
What is biliverdin?
a green pigment resulting from hemoglobin breakdown
What is urobilin?
yellowish pigment that is responsible for the color of urine