soil science test 4
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
How much CO2 and O2 in the soil air realative to the atmosphere.
soil air has higher CO2 and Lower O2
What is oxygen availavility determined by? (O2 rates of soil) Soil Air
Macroporosity- More macropores= more easily O2can be replenished = more O2 available
Water content- Less water = fewer blocked pores for gas exchange = more O2available
/\ these 2 together make Air-filled porosity
O2 consumption rate- (i.e., number of organisms in soil)
• Fewer organisms = less O2consumption for respiration = more O2available
What 4 things happen with excess water in soil?
-anaerobic conditions
-Some nutrients become less available
-some pollutant toxicity changes
-soil color becomes more grey
Specific heat
-Amount of energy to heat soil
-SpH of water > soil> soil air
-wet soils are harfer to heat than dry ones
-wet soils store more heat
Thermal conductivity
-movement of heat through soil
-(highest) Soil> water> soil air
-particles with close proximety conduct heat
-water conducts heat
-wet compacted soils conduct heat well
Temperature moderation and depth
-The deaper the soil, the less seasonal temp change.
-Depth delays T changes
Out of micropores and macropores what is likly to be filled with what?
Macropores are more likly to be folled with air
since micropores are filled with water
How to improve aeration in soil?
-improve aggrgation ( add calcium for flocculation)
-improve tillage
-reducing compaction
What can be slowed by lower temperature?
Plant growth
plant uptake of water
decomposistion of OM
Nutrient cycling
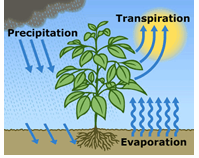
Hydolic cycle
process of cycling water from warth to atmosphere and back
What is evaporation and transpiration
evaporation Conversion of liquid water into water vapor
transpiration process of water movement in a plant and eventual evaporation from the leaf to the atmosphere
eveotranspiration.
Water balance equation
P = ET + SS + D
P = precipitation (may also represent irrigation)
ET = evapotranspiration- Depends on vegetation, soil cover, temperature, humidity
SS = soil storage- Depends on infiltration rate, soil moisture state, topography, microrelief
D = discharge (drainage or runoff)
What causes run off?
•Depends on
1• Precipitation intensity & duration (timing of snowfall) •Vegetation type
• Soil properties
1• Porosity, BD, compaction, Texture/structure
2• Water holding capacity
3• Temperature/Season
How to encourage infiltration?
• Cover crops
• Conservation tillage
• Strip cropping
• Furrow dikes
• Contour rows
• Terraces
•Any process that increases OM and improves structure
• Subsurface drainage
Potential Evapotranspiration (PET)
Amount of water vapor that woild be lost frim a densly vegetated soil IF soil water content were optimum.
What does PET Potential evapotranspiration depend on?
-temperature
-relative humidity
-cloud cover
[-wind
Transpiration efficiency
Dry matter yeild/unit water lost to T
ET efficency
dry matter yeild per unit water lost to ET
Transpiration ratio
kg water transpired per 1 kg dry matter biomass
How to control ET? evap and transpiration
•Manage crop
•(Irrigation)
•Limit plant nutrients
•Sow fewer seeds
•Plant so there is some kind of shading
•Eliminate weeds
•Terminate cover crops sufficiently early
•Mulch
Factors that affects leaching
-Solubility
-Texture, structure
-Frequency & Amount of percolate
-soil pH
-Soil fertility status
What is a consequence of excess runoff (leaching to water?
Eutrophication
What are the 2 ways to remove excess water? and the pros and cons
Surface drainage
-Handles large volumes
-Low installation cost
-More prone to erosion
-High maintenance cost
Subsurface drainage
-Provides percolation
-low maintenance cost
-high rainfall can lead to leaching and flooding
-high installation costs
Total water potential = ?
Gravitational, matric, and osmotic
What are the 4 types of colloids?
Phyllosilicate clays, Fe/Al oxides, humus, Allophane
What are the properties of a colloid?
-small size
-Large surface area
-typically negatively charged
What 5 minerals are Phyllosilicates?
smectite, vermiculite, mica, chlorite, kaolinite
What Phyllosilicates are 1:1 and 2:1
Kaolinite = 1:1, smectite, vermiculite, mica, chlorite = 2:1
Which Phyllosilicats are expanidible?
Smectite & vermiculite
Interlayers of each of the phyllosilicates Kaolinite Mica Chlorite Smectite Vermiculite
• Kaolinite = H-bonds
• Smectitite and Vermiculite = hydrated cations
•Mica = K+
• Chlorite = Mg-oxide
Phyllosilicates with a pH dependat souce charge. and how is it caused?
• All colloids
• Caused by broken edges that can protonate based on pH
Phyllosilicates with a permanent source charge? and how is that caused?
• Only 2:1 colloids (smectite, vermiculite, mica, chlorite)
• Caused by isomorphic substation (substitution of one
cation for another in the crystal structure)
Order these in CEC of kaolinite, smectite, mica, vermiculite, Humus Fe/Al oxides, chlorite
Humus > smectite/vermiculite > mica/chlorite > kaolinite >
Fe/Al oxides
CEC defined as
“the sum total of the exchangeable cations that a soil can absorb
(it reprensents the number of charges NOT the number of ions)
Important cations
Al, Ca, Mg, K, Na, H
What is cation adsorption determined by?
strengh of adsorption; Al3+ > Ca2+ > Mg+2 > K+ = NH4+ > Na
concentration in solution
CEC exchange properties
rapid, reverseable, charge based.
Regional effect of CEC
•Humid regions – higher weathering, more Al and H (acidic)
•Arid regions – lower weathering, more basic cations
CEC of colloids (specific numbers not necessary, but relative to one another)
Which has the most CEC?
•Humus > smectite/vermiculite > mica/chlorite > kaolinite > Fe/Al oxides
CEC conversion rates
1 mol= 100 cmol= 1000mmol
cmolc = cmol * charge of the ion
mg to mmol- mmol = mg / Atomic Weight (AW)mg
How to estimate the CEC from cations?
add up the cations values to get the estimate CEC
How to estimate the CEC from the mineral composistion of the soil?
Weighted average
How to calculate base sateration from exchangeable cations
%BS= (CEC due to Ca, Mg, K, Na,/CEC) *100
only the bases mentioned above. Add them together. and divide by the CEC of everything including the nonbases.
Anion exchange capacity
Similar to CEC, except with anions
• Represents the exchangeable anions adsorbed on soi
not commonlly used
most soils have low or no AEC
Which clay particles will always exhibit some CEC? Why?
Anything with permeant charge (2;1 clays)
Which clay particles are likely to have AEC when pH is low? Why?
iron alumion oxides
Definition ph
• Negative log of hydrogen ion activity or concentration in solution
•How does pH affect soil
Plant and microbial growth
• Nutrient availability/toxicity
• CEC
• OM formation/decomposition
• Fate of pollutants
• Aggregate stability
• Movement of air/water
pH Dependent Charge only
1:1
• Oxides
• Humus
Dominated by some Permanent Charge
(PC mostly neutralized)
• Mica
– Neutralized by K+ in interlayer
• Chlorite
– Neutralized by Mg-oxide layer in interlayer
Dominated by Permanent Charge
– Vermiculite
• Moderately high permanent charge
• Exchangeable cations neutralize charge
• Shrink-swell
– Smectite
• Moderate permanent charge
• Exchangeable cations neutralize charge
• Shrink-swell
the principal source of negative charge on smectite is due to?
-isomorphic substitution


The majority of the charge on 1;1 aluminosilicates minerals arise from?
hydrogen dissosciation from particle edges
Active acidity
H+ activity in the soil solution
Exchangeable Acidity
Exchangeable Al3+ and H+
• Released by CEC reactions
Residual acidity
Al and H that are components of soil
particles
• E.g., H+ from pH dependent charge
• E.g., Al released as mineral weathers
pH Buffering
pH > 7
• Carbonates
pH 5.5 to 7
• Surface-bound Al
• Reflected by CEC
pH 3.5 to 5.5
• Exchangeable Al3+ and H+
pH < 3.5
• Free strong acid production (FeS2 H2SO4)
pH > 7
Poorly availability micronutrients (Fe, Zn, Cu, Mn)
• Usually soils are calcareous/have CaCO3
pH 5.5 to 7
• Maximum P availability
pH 3.5 to 5.5
• Exchangeable Al3+ can be toxic
• Plants have difficulty with nutrient uptake
pH < 3.5
• Exchangeable Al3+ can be toxic
• Plants have difficulty with nutrient uptake
Al is more acidic than H
More Al = more acidity
•Ratio of Al to H
• 10:1 is more acidic than 1:10
acidity and salts
Neutral salts typically lower pH
•Salts can appear by
• Fertilization
• Seasonal differences in leaching
name a couple weak acids
• Carbonic acid
• Rainfall
• Respiration
• Acid rain
• Root/microbial exudates (organic acids)
• Acidic groups on organic matter
•Nutrient sources of acidity
• Nitrification
• Plant uptake of NH4
+ and basic cations
• Leaching of basic cations
What can caise acidity in crop production?
• Crop removal
and fertilizers
What kind of minerals can cause acidity?
sulfur compounds
Reactions that consume acidity
(i.e., promote higher pH)
Nitrate
• Plant uptake
• Denitrification
Weathering of soil minerals
• Release of basic cations
Climate
• When ET > rainfall
Common types of lime
Calcitic lime
• Dolomitic lime
• Burned lime/quicklime
• Oxides of Ca and Mg
• Hydrated lime
• Hydroxides of Ca and Mg
•Reaction of lime in soil
CaCO3 +H2O → Ca2+ + CO3 2-
Ca2+ displaces any exchangeable acidity
• Carbonate reacts with H+ and eventually forms CO2 and water and is release
Neutralizing power of CaCO3
1000 lb/a CaCO3 will neutralize 1 cmolc/kg acidity (AFS)
• ppmx2 = pp2m = lb/a
• mg/kg = ppm
What is CCE?
Neutralizing power in terms of CaCO3
reflects acid neutralizing strength of material
What is ECCE
ECCE
• Based on CCE and particle size
• Reflects actual neutralizing ability
Lime requirement depends on
Initial pH
• Target pH
• Buffer capacity of soil (i.e., how much exchangeable/residual acidity need to be
neutralized to actually see a change in pH)
• Depth of pH adjustment
• Properties of liming material
• CCE, particle size (or ECCE)
Application of lime fequency timing
Frequency
• 2-5 yr
Timing
• 6 to 12 mo prior to desired pH change
Incorporation vs surface application Subsurface acidity
• Consider CaSO4 to detoxify Al and add Ca to
Arid/semi-arid soils are usually…
Water limited
• Often carbonate-rich
• Often affected by poor leaching of salts
• ET > rainfall
• Typically have pH > 7
• Potential problems with micronutrient deficiencies (Fe, Mn, Cu, Zn)
• Potential problems with P and B deficiency
• Molybdenum is usually high
• CEC is usually high
• Usually considerable 2:1 clays
• pH increases pH dependent charge
• Usually high %BS, due to lack of leaching of base cations
What is the source of salts?
Weathering of rocks/minerals
• Fossil deposits
• Irrigation
• Poor drainage
Assessing Soil for Salts
Salinity
• Measures total salts
• Electrical conductivity
Sodium
• Exchangeable Na (ESP)
Saline
Saline
EC 4 or above
• High salts – Ca, Mg salts primarily
• Plants struggle to grow
Sodic
Sodic
ESP 15 or above
• High Na
• Low salinity
• pH > 8.5
• Poor infiltration, plants struggle
with lack of water
Saline-Sodic
Saline-Sodic
EC 4 and ESP 15 or greater
• High Na
• High salinity
• Plants struggle to grow
• Can be transformed to sodic
Reclamation of Saline soils
Leaching
• Ensure water is low Na
Drainage
• Add drainage (stop evaporatiom)
• Manage drainage waters
Monitor EC
• Before, during, and after
Reclamation of Saline-Sodic
& Sodic soils
Apply Ca
• Gypsum
Leaching
• May need ponding for sodic soils
Drainage
• Add drainage
• Manage drainage waters
Monitor EC & ESP
• Before, during, after