CHEM 4
0.0(0)
Card Sorting
1/111
Earn XP
Description and Tags
Last updated 9:51 AM on 11/16/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
112 Terms
1
New cards
What is an ionic bond
- metal cation bonded to a non-metal anion
- A chemical bond formed between two ions with opposite charges
- the uneven sharing of electrons
- A chemical bond formed between two ions with opposite charges
- the uneven sharing of electrons
2
New cards
Why are the atoms charged in ionic bonds?
metal atoms lose electrons to non-metallic atoms so they become positively charged metal ions
non-metals gain electrons from the metal atoms and become negatively charged non-metalions
non-metals gain electrons from the metal atoms and become negatively charged non-metalions
3
New cards
How does an ionic compound form?
a large number of positive and negative ions combine to form a 3D giant ionic lattice structure
4
New cards
How does the 3D lattice stay together?
held together strongly by electrostatic forces of attractions between the positive and negative ions (aka ionic bonding)
5
New cards
Why is the mpt and bpt high in ionic compounds?
due to the strong electrostatic forces of attractions
6
New cards
Standard conditions for ionic compounds
- Ionic substances are solid
- need to be dissolved in water to make a solution
- need to be dissolved in water to make a solution
7
New cards
3D Giant ionic lattice drawing
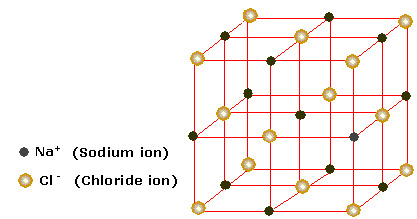
8
New cards
what are electrostatic attractions? (definition)
the attraction of oppositely charged ions
9
New cards
Ionic bonding diagram (Na & Cl)
Na wants to loose 1 electron and chlorine wants to gain one
Na will transfer its electron to the chlorine to become a sodium cation and a chlorine anion
Na will transfer its electron to the chlorine to become a sodium cation and a chlorine anion
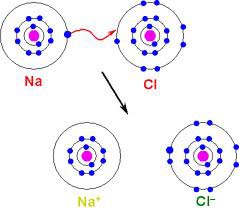
10
New cards
What do the brackets mean by transition metals? eg: Iron(III)
Since transition metals have multiple oxidation states we must write the roman numerals in brackets to indicate which one it is
11
New cards
-IDE
the element is a monoatomic ion (by itself)
12
New cards
-ITE
contains oxygen, but less than ate
13
New cards
-ATE
contains oxygen, more than ite
14
New cards
What are the ionic properties
Volatility
Electrical conductivity
High MPT
Solubility
Electrical conductivity
High MPT
Solubility
15
New cards
What is volatility?
the tendency of a substance to turn into gas (to vaporise)
16
New cards
Volatility in ionic compounds?
low volatility/non-volatile
17
New cards
Why do ionic compounds have low/none volatility?
SOLID: Because they have strong electrostatic attractions between ions in the ionic lattice
SOLUTION: When dissolved in water they ions have strong attractions with water molecules
= hard to break attractions
SOLUTION: When dissolved in water they ions have strong attractions with water molecules
= hard to break attractions
18
New cards
Electrical conductivity in ionic compounds?
SOLID: no
SOLUTION: yes
SOLUTION: yes
19
New cards
Why is the electrical conductivity trend in ionic compounds the way it is?
SOLID: cannot conduct electricity because ions are not moving freely (held by electrostatic attractions) so there is no flow of charge which is necessary for conducting electricity.
SOLUTION: ions are free to move and therefore they can conduct electricity (migration of ions = current)
SOLUTION: ions are free to move and therefore they can conduct electricity (migration of ions = current)
20
New cards
What is needed to conduct electricity?
A flow of charge
21
New cards
Why is there a high MPT of ionic compounds?
strong force of electrostatic attractions in the lattice between ions = a large amount of heat (energy) needed to break those attractions
(PS: higher melting points when the charge on the ions are greater because there is an increased attraction between the ions)
(PS: higher melting points when the charge on the ions are greater because there is an increased attraction between the ions)
22
New cards
What is solubility?
the ease at which a solute dissolves in a solvent to form a solution
23
New cards
Solubility in ionic compounds?
High solubility
24
New cards
Why are ionic compounds soluble? (eg NaCl)
when NaCl has dissolved in water the sodium and chloride ions are surrounded by water molecules
The ions are hydrates as new bonds form between ions and the water molecules (ion-dipole attractions)
The ions are hydrates as new bonds form between ions and the water molecules (ion-dipole attractions)
25
New cards
Diagram of ionic substances dissolved in water (NaCl)
When dissolved positive and negative ions in the lattice separate because the solvent pulls the lattice apart
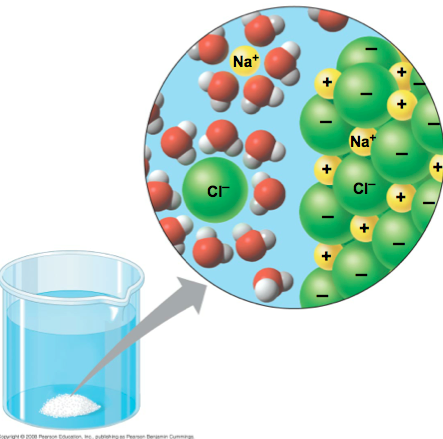
26
New cards
What determines how ionic a compound is?
electronegativity:
the greater the electronegativity difference of the atoms in a compound the more uneven the sharing of electrons is (more likely to be ionic)
the greater the electronegativity difference of the atoms in a compound the more uneven the sharing of electrons is (more likely to be ionic)
27
New cards
What is a covalent bond?
the electrostatic force of attraction of one or more pairs of shared electrons to the 2 nuclei they are shared between
28
New cards
Covalent molecules: random facts
Share electrons to gain a full outer shell
Follow the octet rule
Covalent compounds have no free electrons and no ions so they don't conduct electricity
Follow the octet rule
Covalent compounds have no free electrons and no ions so they don't conduct electricity
29
New cards
How does a covalent form?
Forms when outer electrons come close enough to each other to interact and rearrange themselves into a more stable arrangement
30
New cards
A double bond is ... than a single bond. finish the sentence and why?
shorter and stronger because there is a greater attraction between the nuclei and shared electrons pulling them closer
31
New cards
Bond length
a measure of the distance between the two bonded nuclei
32
New cards
Bond strength
described in terms of bond enthalpy (KJ/mol) - a measure of the energy needed to break a bond
33
New cards
Bond length trend
as the atomic radius increases as we move down a group atoms form molecules with longer bonds
34
New cards
non-polar covalent bond
when two atoms with the same electronegativity share an electron pair
35
New cards
polar covalent bond
when atoms with different electronegativity values share an electron pair
- the atom with the larger electronegativity draws the electrons pair closer to the nucleus making a polar covalent bond
- the atom with the larger electronegativity draws the electrons pair closer to the nucleus making a polar covalent bond
36
New cards
When comparing covalent bond - what to mention?
talk about strength
talk about length
what type of bond
Bond order
talk about length
what type of bond
Bond order
37
New cards
what does the number of covalent bonds that an atom will form depend on?
number of electrons needed to fill its shell (look at periodic table)
38
New cards
What is the octet rule?
the tendency of atoms to gain a valance shell with a total of 8 electrons
39
New cards
Exceptions to the octet rule?
Period 3 and below can sometimes expand their octet
B & Be - don't need 8 electrons
S - can expand its octet
B & Be - don't need 8 electrons
S - can expand its octet
40
New cards
NH3
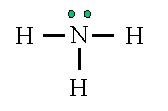
41
New cards
H20
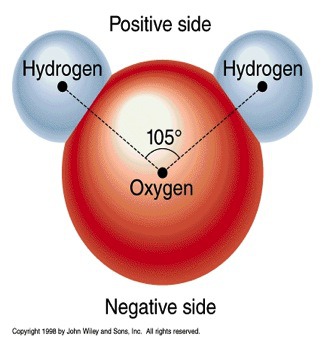
42
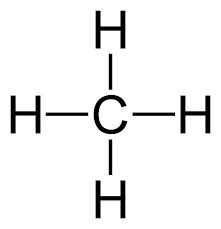
New cards
CH4
43
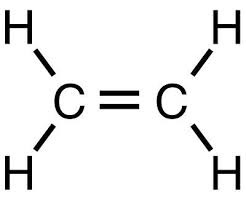
New cards
CO2
44
New cards
HCN
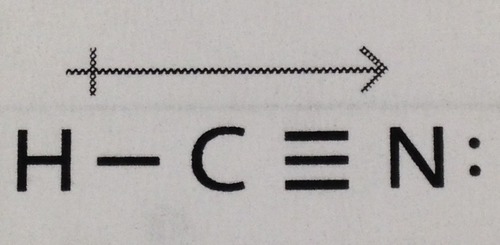
45
New cards
BeCl2
(stable with 4 electrons in the outer shell)
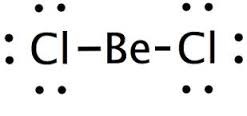
46
New cards
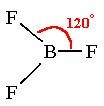
BF2
(stable with 6 electrons in the outer shell)
47
New cards
What are electron domains?
refers to pairs of electrons on a central atom (both non-bonding and bonding)
48
New cards
What do the number of electron domains determine?
the shape of the molecule as a result of electron repulsion
49
New cards
How does electron repulsion work?
each pair of electrons is repelled as far as possible from each other
50
New cards
Dative/coordinate bond
a covalent bond in which both the shared electrons are provided by one of the atoms
51
New cards
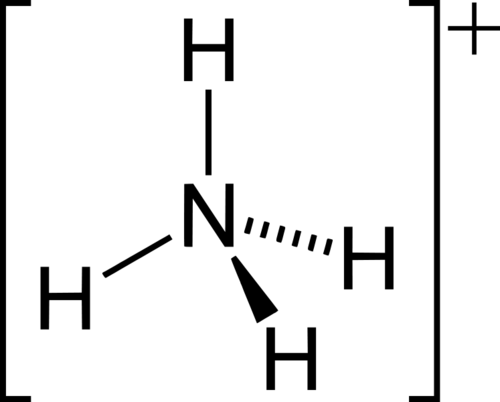
NH4+ (polar?)
arrow pointing towards the top hydrogen atom
52
New cards
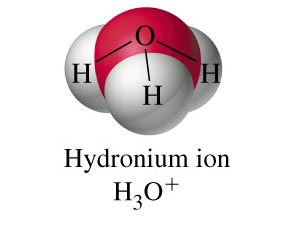
H3O+ (polar?)
Arrow pointing towards a hydrogen atom
53
New cards
What is molecular geometry?
Explains the 3D shape of the molecule
54
New cards
When is a molecule polar?
1) when theres a difference of electronegativity between atoms
2) Asymmetrical distribution of partial charge (one positive and one negative end, the charges do not cancel out)
2) Asymmetrical distribution of partial charge (one positive and one negative end, the charges do not cancel out)
55
New cards
What is a resonance structure?
A method of describing the delocalized electrons in some molecules where the bonding cannot be explicitly expressed by a single Lewis structure.
56
New cards
Why do resonance structures exist?
-when electrons can move between parts of the molecules
-since we dont know which position of the double bond is more common
-since we dont know which position of the double bond is more common
57
New cards
Resonance structures examples
Ozone - 03
Carbonate - CO3 2-
Nitrate - NO3-
Carbonate - CO3 2-
Nitrate - NO3-
58
New cards
Carbon Molecules can be in the form of
- Allotropes - diamond, graphite, fullerene which contain carbon but vary in structure
59
New cards
Graphite (6 points)
- hard in one direction soft in the other
- layered structure, weak LDF between layers
- carbon atoms held together by very strong covalent bonds (each carbo has three bonds with 1 delocalised electron pair shared in layers)
- structure = covalent layer lattice
- MG = trigonal planar
- BA = 120
- layered structure, weak LDF between layers
- carbon atoms held together by very strong covalent bonds (each carbo has three bonds with 1 delocalised electron pair shared in layers)
- structure = covalent layer lattice
- MG = trigonal planar
- BA = 120
60
New cards
Dimond
- hardest naturally occurring substance and doesn't conduct electricity
- Carbon is covalently bonded to 4 others with very strong bonds
- structure = a covalent lattice
- MG = tetrahedral
- BA = 109.5
- Carbon is covalently bonded to 4 others with very strong bonds
- structure = a covalent lattice
- MG = tetrahedral
- BA = 109.5
61
New cards
Fullerenes (eg: footballs)
- covalent bonding between 3 carbon atoms
- not in a lattice structure, penta/hexagon shape
- conducts electricity
- not in a lattice structure, penta/hexagon shape
- conducts electricity
62
New cards
About Silicone
- can form 4 covalent bonds
- can form covalent lattice with other silicone atoms
- can form covalent lattice with other silicone atoms
63
New cards
Si-Si compared to C-C
- longer and weaker
- more reactive
- easier to break
- more reactive
- easier to break
64
New cards
What are the different intermolecular forces?
London dispersion forces, dipole-dipole, hydrogen bonging
65
New cards
Relative strengths of the intermolecular forces?
(weaker, low mpt) LDR < diple-dipole < hydrogen bonging (stronger, high mpt)
66
New cards
Intramolecular forces include
(internal, between atoms in the molecule)
- polar covalent
- non-polar covalent
- ionic bonds
- metallic bonds
- polar covalent
- non-polar covalent
- ionic bonds
- metallic bonds
67
New cards
Intermolecular forces include
(bonding between 2 or more molecules)
- LDR
- dipole-dipole
- hydrogen bonding
- LDR
- dipole-dipole
- hydrogen bonding
68
New cards
What is a dipole?
formed when 2 non-metals form a polar covalent bond
69
New cards
What is a dipole-dipole attraction?
the attraction of 2 polar molecules to each other
70
New cards
Strength of Dipole-dipole attractions?
the strength depends on distance and orientation of the dipoles
71
New cards
Example of dipole-dipole attractions in HCl
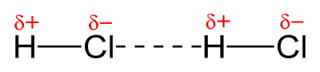
72
New cards
London Dispersion Forces?
present in all molecules
73
New cards
Strength of LDF
as the number of electrons in the molecule increases the LDF become stronger and MPT increases
74
New cards
Instantaneous Dipoles?
Atoms with same electronegativities do not have permanenet dipoles; however they do have instantaneous dipoles due to the constant movement of the electrons
75
New cards
What is hydrogen bonding?
an electrostatic attraction between two polar groups that occurs when a hydrogen (H) atom, covalently bonded to a highly electronegative atom such as nitrogen (N), oxygen (O), or fluorine (F),
76
New cards
Hydrogen bonding occurs when bonding with what three elements?
N-H
O-H
F-H
- because of their big electronegative difference there will be strong hydrogen bonds
O-H
F-H
- because of their big electronegative difference there will be strong hydrogen bonds
77
New cards
What intermolecular forces do polar molecules have?
- LDF
- Dipole- Dipole
- SOMETIMES hydrogen bonding
- Dipole- Dipole
- SOMETIMES hydrogen bonding
78
New cards
What intermolecular forces do non-polar molecules have?
- only LDF
- instantaneous dipoles may occur
- instantaneous dipoles may occur
79
New cards
When are covalent molecules soluble?
If they can form bonds with the solvent
80
New cards
Non-polar solvents:
C6H12
81
New cards
Why is water such a good solvent?
Very polar, forms dipole-dipole/hydrogen bonds, pulls apart ionic compunds
82
New cards
what does metallic bonding consist of?
positively charged ions arranged in a regular lattice held together by electrostatic attractions of the cations and the delocalised electrons
83
New cards
Diagram for metallic bonding
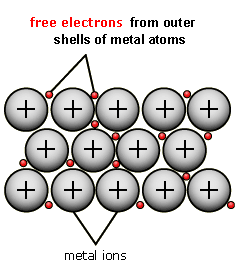
84
New cards
About the delocalised electrons in metallic bonding
constant random motion
between the ions
came from outer shells of metals
free to move
between the ions
came from outer shells of metals
free to move
85
New cards
Properties of metallic bonding?
Malleability
Electrical conductivity
Electrical conductivity
86
New cards
Explain Malleability of metals
it enables metals to be pressed into any shape
- metallic bonding: the layers of ions can slide over each other while remaining connected to the sea of electrons and therefore it doesn't break but changes shape
- metallic bonding: the layers of ions can slide over each other while remaining connected to the sea of electrons and therefore it doesn't break but changes shape

87
New cards
Explain electrical conductivity of metals
There are free flowing delocalised electrons, a flow of electrons and they can therefore conduct electricity
88
New cards
How can properties of metals be altered by adding other substances?
other substances are added and (usually other metals or carbon) melted together with the metal and cool so the atoms mix (the resulted solid is called an alloy)
89
New cards
How is it possible to add other substances to metals?
because of the non directional nature of the delocalised electrons and that the lattice can accommodate ions of different sizes
90
New cards
Which is stronger; Ionic or covalent compounds?
Ionic because electrostatic attractions are stronger than the sharing of electrons
91
New cards
What are substitutional alloys?
made from addition of elements with similar chemical properties and atomic size
Still a strong lattice structure
The size difference between the atoms causes a restriction between the layers (harder and less malleable)
Still a strong lattice structure
The size difference between the atoms causes a restriction between the layers (harder and less malleable)
92
New cards
What are interstitial alloys?
Addition of a smaller atom to a metal
fits randomly between packed metal ions
causes more restriction = less malleable
fits randomly between packed metal ions
causes more restriction = less malleable
93
New cards
Why is formal charge important?
allows us to determine the most stable and likely lewis structure
94
New cards
Equation for formal charge
FC= V-L-B/2
V- total number of valance electrons (P-table)
L- lone pair of electrons (the free ones around the atoms)
B- bonding electrons (in the bonds)
V- total number of valance electrons (P-table)
L- lone pair of electrons (the free ones around the atoms)
B- bonding electrons (in the bonds)
95
New cards
How do you know which lewis structure is the most stable?
The one with a formal charge closest to 0
The negative charges are located on the most electronegative atoms
The negative charges are located on the most electronegative atoms
96
New cards
How to calculate bond order of resonance structures?
# of bonds / # of possible locations of bonds
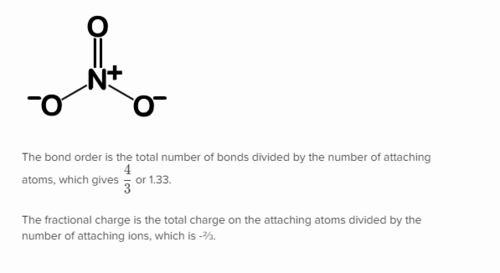
97
New cards
What is meant by a bond order of 1.5?
Bond is stronger than a single bond but weaker than a double bond
98
New cards
What are the three basic concepts of orbital overlap theory?
1) the orbitals occupy the same space
2) larger overlap = stronger bond
3) a pair of electrons is located at an overlap
2) larger overlap = stronger bond
3) a pair of electrons is located at an overlap
99
New cards
What are the two types of covalent bonds?
sigma and pi bonds
100
New cards
When do sigma bonds occur?
axial - between the atomic nuclei