Untitled
0.0(0)
0.0(0)
Card Sorting
1/104
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
105 Terms
1
New cards
What is electronegativity?
How strongly atoms attract bonding electrons to themselves.
2
New cards
If a bond is polar?
It has a dipole moment
3
New cards
If Nitrogen, Florine, Oxygen, or Chloride is bonded to a non-metal other than itself
Then the bond will have a dipole moment
4
New cards
Hydrogen bond donators
Are hydrogen atoms attached to N or O, they play a key role in hydrogen bonding with water
5
New cards
Non polar covalent
equal sharing of electrons ( Cl2, CS2, or C-H)
6
New cards
Polar covalent
unequal sharing of electrons (NH3, H2O)
7
New cards
Ionic Bond
Formed when one or more electrons are transferred from one atom to another. ( NaCl or MgCl2)
8
New cards
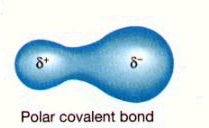
Polar covalent bond
δ+ (the atom with the lesser share of electrons), δ-
(the atom with the greater share of electrons)
(the atom with the greater share of electrons)
9
New cards
Will a molecule always have a dipole moment even if it contains polar covalent bonds
No, always look at its geometry
10
New cards
Hydrophilic compounds
Compounds that interact with water are polar or carry an electric charge (positive or negative)
11
New cards
Lipid soluble (lipophilic)
Are water-insoluble (non-polar) Ex: hydrocarbons.
12
New cards
Delta H(enthalpy)
exothermic, energetically favorable
13
New cards
Negative delta G
spontaneous, exergonic, starts the reaction in a compound
14
New cards
Positive delta S
more disorder, hydrophobic effect
15
New cards
S character
sp3 = 25%, sp2 = 33%, sp = 50% (remaining percent is p character)
16
New cards
Amphipathic
having both a hydrophilic (polar) region and a hydrophobic (non-polar) region
17
New cards
Why are drugs Amphipathic?
In order to pass through the cell membrane bilayer (non-polar) and then dissolve in water (polar).
18
New cards
Phospholipids
A molecule that is a constituent of the inner bilayer of biological membranes, having a polar, hydrophilic head and a nonpolar, hydrophobic tail.
19
New cards
Individual water molecules forming a cage have less...
entropy (disorder) b/c they are more highly organized.
20
New cards
Acid
compound that donates a proton (H+) to water
21
New cards
Base
A compound that can accept a proton from water (BH+)
22
New cards
Protonated (P)
the compound with the extra hydrogen atom
23
New cards
Unprotonated (U)
The compound with one less hydrogen atom
24
New cards
If the neutral (unionized) specie of the pair (P and U) is donating (losing) a proton it would be considered...
An acid
25
New cards
If the neutral (unionized) specie of the pair (P and U) is accepting a proton it would be considered...
A base
26
New cards
A weak acid and base...
will not ionize 100%, both the protonated and unprotonated compounds will co-exist in the solution
27
New cards
Which is the protonated compound in this example?
CH3COOH + H2O = (CH3COO-) + (H3O+)
CH3COOH + H2O = (CH3COO-) + (H3O+)
CH3COOH, b/c it starts with the extra H+ and its also considered neutral since it doesn't have a charge.
28
New cards
Which is the unprotonated compound in this example?
CH3COOH + H2O = (CH3COO-) + (H3O+)
CH3COOH + H2O = (CH3COO-) + (H3O+)
CH3COO- , b/c it donated its H+ to water
29
New cards
Organic acids and bases are generally classified as...
WEAK, they do not ionize completely.
30
New cards
The larger the numerator in a Ka/Kb means...
the acid or base is stronger, it produces more products (ionized), more acidic/ basic
31
New cards
The smaller the pKa value of an acid means...
the stronger the acid
32
New cards
The smaller the pKb value of a base means...
the stronger the base
33
New cards
pKa + pKb =
14
34
New cards
pOH + pH =
14
35
New cards
The Golden Equation
P/U = 10^(pKa - pH)
For acids: P = HA (unionized) , U = A- (ionized)
For bases: P = BH+ (ionized) , U = B (unionized)
For acids: P = HA (unionized) , U = A- (ionized)
For bases: P = BH+ (ionized) , U = B (unionized)
36
New cards
Golden Equation for an acid:
HA/A-
37
New cards
Golden Equation for a base:
BH+/B
38
New cards
The LARGER the "pKa" value listed for a BASE...
the STRONGER the BASE
39
New cards
Where will an acid be more unionized. In the stomach pH= 1.5 or in the blood pH = 7.4 ?
In the stomach b/c the lower pH (more H+ ) of the stomach favors formation of more HA, the protonated form of the acid, which would be formed in the blood.
40
New cards
Lowering the pH of the surrounding environment favors the formation of...
the protonated form (HA/unionized) more than previously.
41
New cards
Absorption through a biological membrane would need...
a compound that is uncharged (neutral) to 100% be absorbed
42
New cards
When the pH is lowered for an acid...
the equilibrium will be driven to the left favoring the formation of HA (protonated).
43
New cards
Adding more protons (lowering the pH of the environment) causes what to a base?
BH+ to form more which is the pronated/charged form of a base. In this case the equilibrium is shifting to the right.
44
New cards
At a higher pH a base will...
favor the unprotonated/uncharged form (B)
45
New cards
Where will a base be more unionized (neutral). In the stomach pH= 1.5 or in the blood pH = 7.4 ?
In the blood b/c at a higher pH the base will become more unprotonated (B) which is the unionized form for a base.
46
New cards
Lowering a the pH in a environment favors...
protonation of either an acid or base relative to the previous pH. HA (unionized) for an acid, and BH+ (ionized) for a base.
47
New cards
pH>pKa
unprotonated will be predominant ( A- for an acid; B for a base)
48
New cards
pH
protonated will be favored (HA for an acid; BH+ for a base)
49
New cards
If both the pH and the pKa are equal...
then there will be equal amount of protonated and unprotonated species present, 10^0=1
50
New cards
An acid in a acid becomes...
Neutral
51
New cards
A base in a base becomes...
Neutral
52
New cards
An acid in a base, or a base in an acid becomes...
Ionized (charged)
53
New cards
Non-polar substances are able to be absorbed through the lumen of the renal tubules back into the bloodstream due to...
The walls of the renal tubules are made up of cells with their lipid member which are more permeable to UNIONIZED (uncharged) molecules.
54
New cards
Acids/Bases in their neutral (uncharged) forms can be...
Reabsorbed
55
New cards
Sodium Bicarbonate (NaHCO3)
can be used to alkalinize the urine.
56
New cards
By raising the pH of the lumen of the renal tubules (where the environment is neutral) with sodium bicarbonate during a overdose of an acidic drug...
We can increase the fraction of the drug in its ionized form (A-) b/c the unprotonated form of the species is favored as a result.
57
New cards
By lowering the pH of the lumen of the renal tubules with ammonium chloride during a overdose of a basic drug...
We can increase the fraction of the drug in its ionized form (BH+) b/c the protonated is favored as a result.
58
New cards
Acids/Bases in their charged (ionized) form...
can not be absorbed by a membrane
59
New cards
An increase in the ionized form of a drug...
decreases REABSORPTION, thus enhancing renal elimination.
60
New cards
Acetylsalicyclic acid (aspirin) can do great harm to the body in overdose, in fact, it can be lethal. Suppose a patient arrives in your hospital's emergency room who has tried to commit suicide by swallowing a whole bottle of aspirin tablets he found in his medicine chest. What might you want to do to detoxify the patient?
Administer sodium bicarbonate to alkalinize (raise pH level) the patient's urine. This will increase the fraction of aspirin in its negatively charged (A- ) form. This form is not reabsorbed from the renal tubule, and will remain in the urine to be excreted. Blood levels of aspirin will consequently decrease
61
New cards
Is Nicotine an acid or base?
A base
62
New cards
Morphine is frequently administered as morphine sulfate. Is morphine an acid or a base?
The negatively charged counter-ion of morphine has "ate" in its name (i.e. sulfate ) soMorphine is a base. Its protonated (BH+ ) is positively charged
63
New cards
Losartan is often administered as "losartan potassium." Is losartan an acid or a base?
The positively charged counter-ion is K+ so Losartan is an acid. Its unprotonated (A- ) is negatively charged
64
New cards
If the name of a salt is sodium valproate, are we dealing with an acidic or a basic drug?
The "sodium" in the name represents "Na+", it is merely the positively-charged counterion in the salt. That means that "valproate" must be negatively, charged meaning it's an acidic drug.
65
New cards
Phenobarbital (weak acid): pKa = 7.4; Acid urine: pH = 5.4
P/U= 10^(7.4-5.4)= 10^(2)= 100.
100x as much unionized (Lipid Soluble (nonpolar) /HA) phenobarbital in the urine which would be reabsorbed
100x as much unionized (Lipid Soluble (nonpolar) /HA) phenobarbital in the urine which would be reabsorbed
66
New cards
Phenobarbital (weak acid): pKa = 7.4; Alkaline urine: pH = 8.4
P/U=10^(7.4-8.4)= 10^(-1)= 1/10.
10x as much unprotonated (lipid insoluble/polar)
A- meaning phenobarbital will be excreted
10x as much unprotonated (lipid insoluble/polar)
A- meaning phenobarbital will be excreted
67
New cards
Kenamycin (weak base): pKa = 7.2; Acidic urine: pH = 6.2
P/U=10^(7.2-6.2)=10^(1)=10
10x as much protonated (lipid insoluble/ polar) BH+ meaning kenamycin will be excreted
10x as much protonated (lipid insoluble/ polar) BH+ meaning kenamycin will be excreted
68
New cards
Kanamycin (weak base): pKa = 7.2; Alkaline urine: pH = 8.2
P/U= 10^(7.2-8.2)=10(-1)=1/10
10x as much unprotonated (lipid soluble/ nonpolar) B meaning kenamycin will be reabsorbed
10x as much unprotonated (lipid soluble/ nonpolar) B meaning kenamycin will be reabsorbed
69
New cards
An organic acid contains a functional group whose...
Neutral form can lose (donate) a proton. Due to the loss of the proton, the substance now bears a negative charge (the anion).
70
New cards
The proton lost in an organic acid will be covalently attached to either...
Oxygen, nitrogen, or sulfur. (O-H, N-H, S-H).
71
New cards
These bonds are considered to be acidic
O-H, N-H, S-H
72
New cards
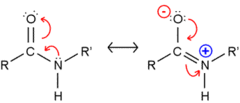
resonance delocalization
occurs in aromatic rings and conjugated double bonds, this stabilizes the compound
73
New cards
To decipher whether a compound is an acid
one should think in terms of the product (conjugate base) resulting from the loss of the proton.
74
New cards
If the negatively charged species (anion) that forms is stable then...
The more stable the negative charge is the stronger the acid.
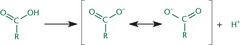
75
New cards
Why is phenol an acid while (alicyclic) cyclohexanol is not?
Due to the resonance delocalization of the negative charge from the oxygen atom of the phenoxide anion increases stability.
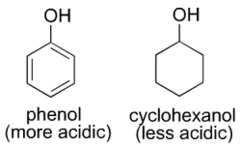
76
New cards
If the hydroxyl group in the cyclohexanol (and most alcohols) were to lose its proton...
then the negative charge on the oxygen atom thats produced will not be able to delocalized meaning there wouldn't be any mechanism to stabilize the charge. Since cychexanol will not give up its proton it's NOT acidic.
77
New cards
resonance stabilization/delocalization
plays a major role in determining whether a compound is acidic or not
78
New cards
An anion that can be further delocalized means...
theres an increase in stability which equals to a stronger acid
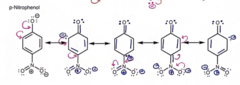
79
New cards
Can you explain why the pKa of picric acid (structure to the right) is so drastically different from that of the other three phenols listed below (i.e., why is picric acid a much stronger acid ?)
Because of the multiple resonance structures that can stabilize the acid
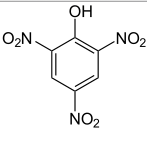
80
New cards
carboxylic acid
is acidic
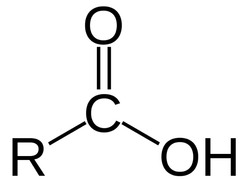
81
New cards
Why are carboxylic acids more acidic than most phenols?
Even though phenols have more resonance structure the negative charge ends on a carbon atom while in a carboxylic acid the negative charge ends on a oxygen atom (more electronegative)
82
New cards
Amides
Should not be considered acidic or basic
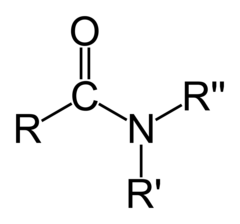
83
New cards
Imides
R1 must be H in order for it to be acidic
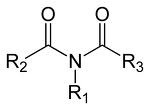
84
New cards
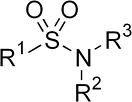
Sulfonamides
R2 or R3 must be H in order for it to be acidic
85
New cards
In the resonance of a sulfonamide...
The negative charge on the N can be delocalized to either of the two oxygen atoms. While this does make it stable most sulfonamide are still weaker than carboxylic acids.
86
New cards
Cyclic Sulfonamides
contains two sulfonamide moieties attached to a ring and in a ring, and is still acidic
87
New cards
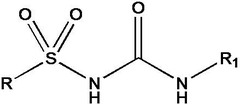
Sulfonylureas
Is more acidic than sulfonamides because once the nitrogen becomes negatively charged it can be delocalized to either oxygen atom attached to the sulfur atom and to the oxygen attached to the carbon atom.
88
New cards
Is sulfonic acid stronger than carboxylic acid? Why?
Yes, because once a negative charge forms on the oxygen atom of the sulfonic acid it can be delocalized to either of the two sulfonyl oxygen atoms making it more stable than carboxylic acid
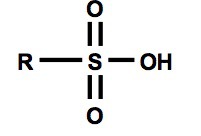
89
New cards
Thiols
Once the thiol loses (donates) its proton the negative charge ends up on the sulfur which is a larger atom than oxygen (spreads over a larger surface area)

90
New cards
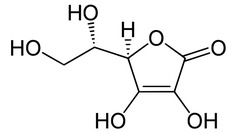
The structure of ascorbic acid is shown below. Note that the compound has two alcoholic OH groups attached directly to the ring. We just stated that aliphatic/alicyclic alcoholics are not acids. Then why is ascorbic acid acidic?
a certain OH group within the molecule is acidic because once this OH group loses (donates) its proton and the negative charge forms on the oxygen atom of the anion, it can be delocalized (and hence, stabilized) via resonance with the carbonyl oxygen.
91
New cards
An organic base...
is a neutral substance that can accept a proton
92
New cards
Usually in medicinal chemistry the atom that accepts the proton is...
a nitrogen atom which bears at least one lone pair of electrons, this forms a positive charge on the nitrogen (N-H)
93
New cards
What can increase the availability of a lone pair?
an increase in electron density (usually on the nitrogen)
94
New cards
Any process that decreases or withdraws electron density from the lone pair of electrons on the nitrogen makes...
the lone pair LESS available to attract and pick up the proton making the substance less basic/weaker
95
New cards
When spotting a nitrogen atom in a compound ALWAYS...
look to see what is immediately bonded to it in all directions. This will determine if the nitrogen atom is a component of an acid, base, or neither.
96
New cards
An aliphatic amine
is an amine in which the nitrogen atom bears a single lone pair and where all the atoms attached to it are either a hydrogen atom or a saturated carbon atom (the carbon has a single bone with all of its substituents)
97
New cards
Depending on how many saturated carbon atoms are attached to the nitrogen , basic aliphatic amines are classified as...
Primary, Secondary, or Tertiary
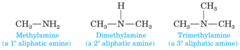
98
New cards
Strength of aliphatic amines (strongest to weakest)
2>1>3 (if, and only if, al three are indeed ALIPHATIC amines
99
New cards
Alicyclic amines
substances in which the nitrogen atom is incorporated into a ring system and all bonds to the nitrogen are hydrogen or a saturated carbon atom. They are BASIC
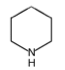
100
New cards
Aromatic amines
are considered very weak bases b/c of an unsaturated carbon atom adjacent to the nitrogen atom, this means the lone pair of electrons on the nitrogen are less available to attract a proton