electrophilic aromatic substitution
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
electrophilic aromatic substitution shortening
SEAr (sub electrophil arom)
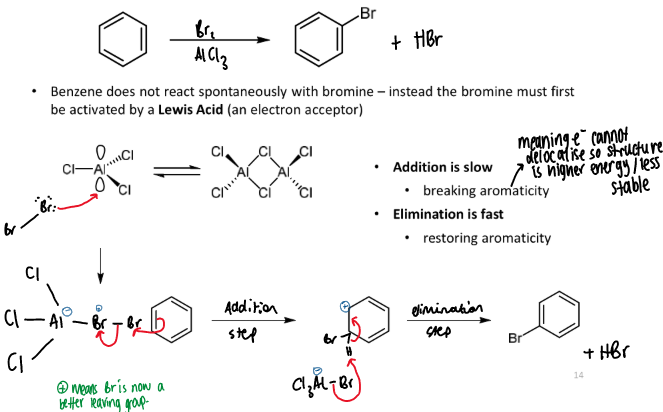
bromination of benzene
overall equation including reagents and conditions
mechanism and why it happens this way
speed of steps in mechanism
mechanism = SEAr
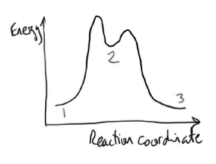
energy profile for bromination of benzene
slowest step = highest energy barrier
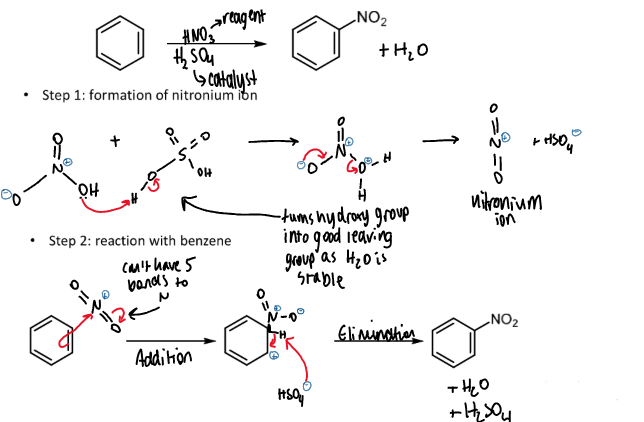
nitration of benzene
overall equation including reagents and conditions
ion needed and how it is formed
mechanism, explanation and names of steps
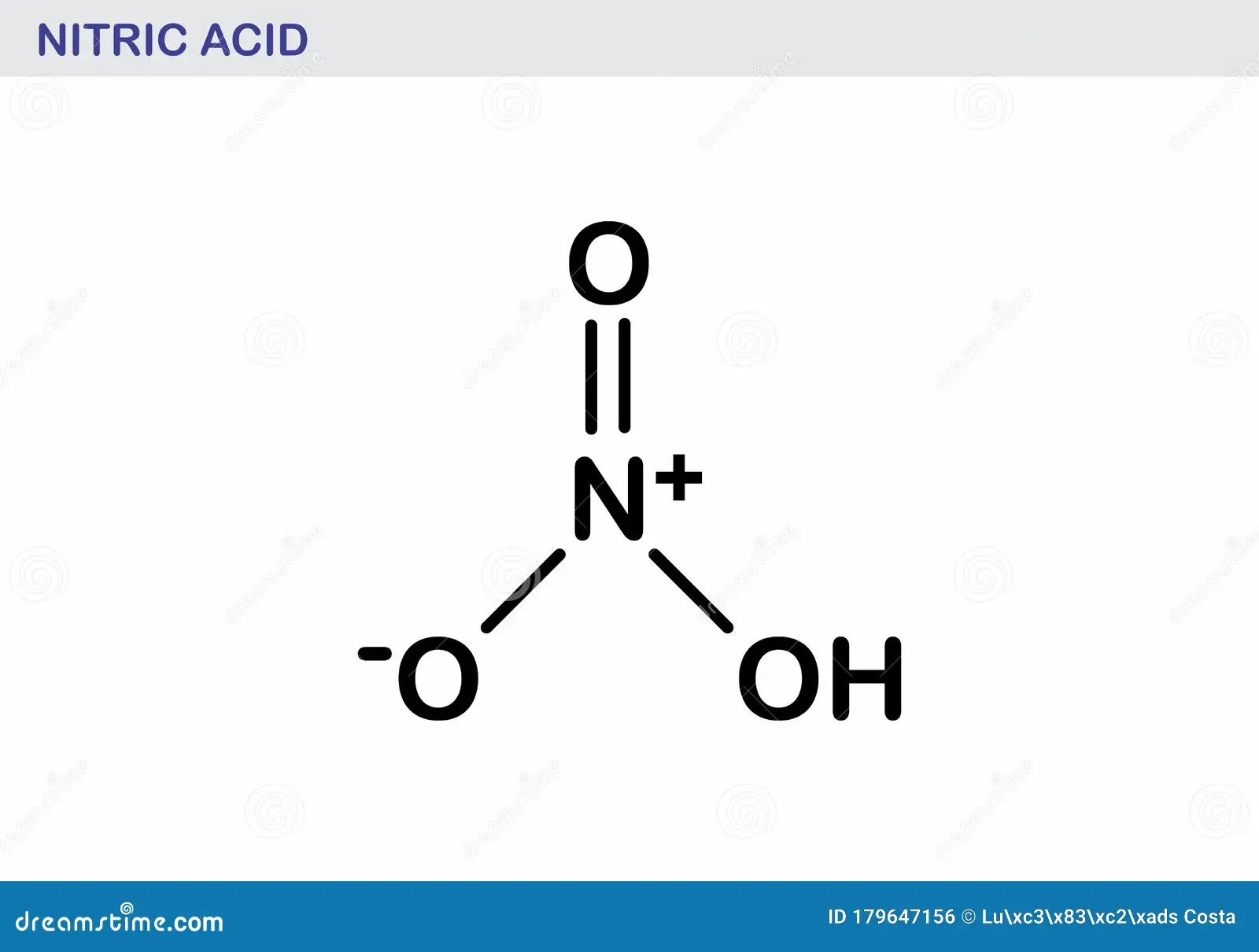
nitric acid structure
HNO3
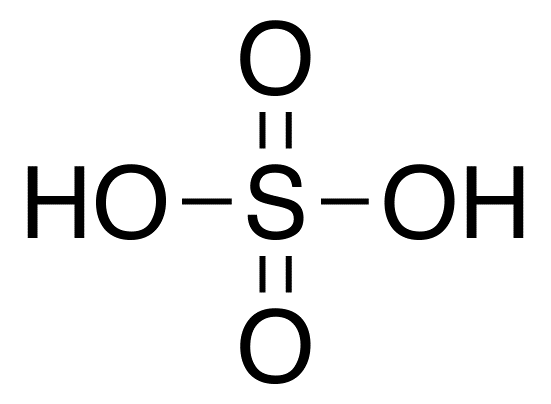
sulfuric acid structure
H2SO4
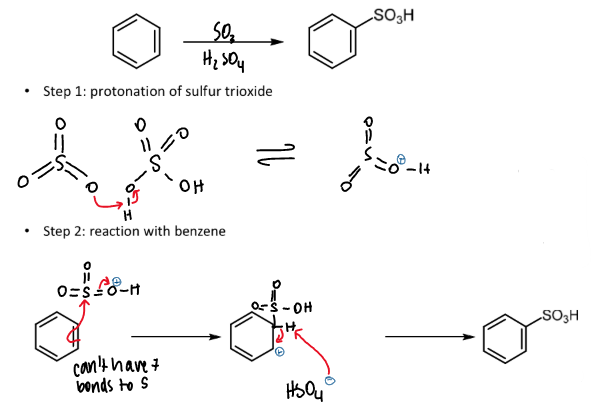
sulfonation of benzene
overall equation including reagents and conditions
mechanism, explanation and names of steps
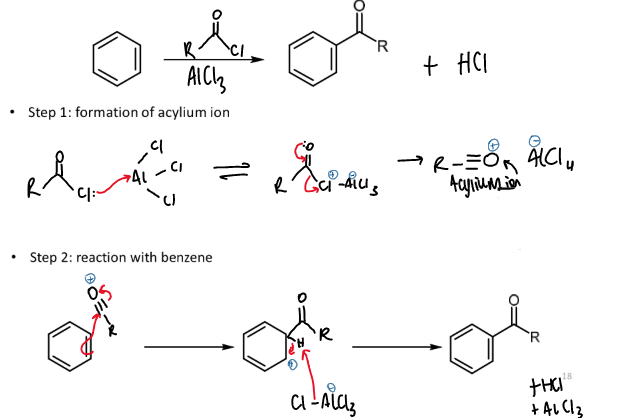
friedel-crafts acylation
overall reaction including reagents and conditions
mechanism and steps
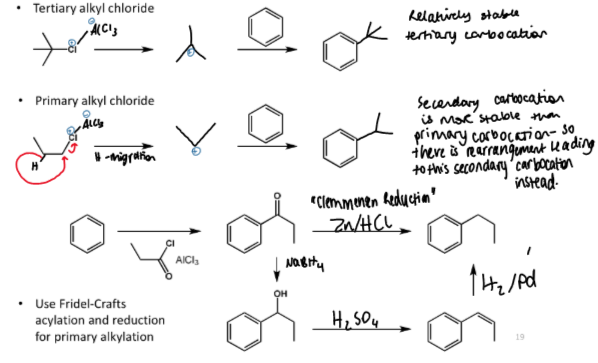
friedel-crafts alkylation
tertiary alkylation
secondary alkylation
primary alkylation
the primary alkylation is not friedel-crafts alkylation but is used as a workaround because primary alkyl chlorides result in rearrangement to secondary carbocations and hence secondary alkylation
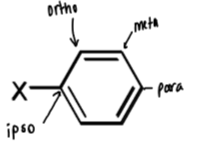
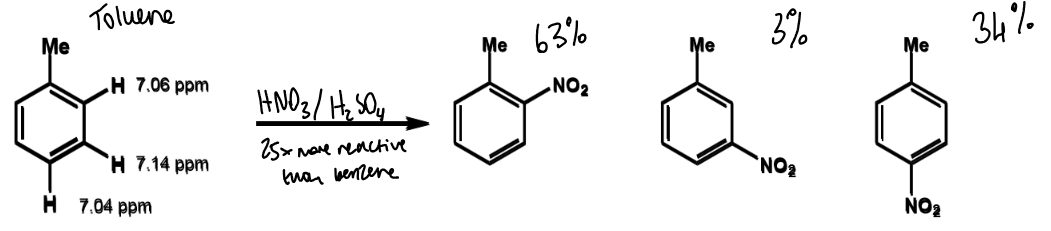
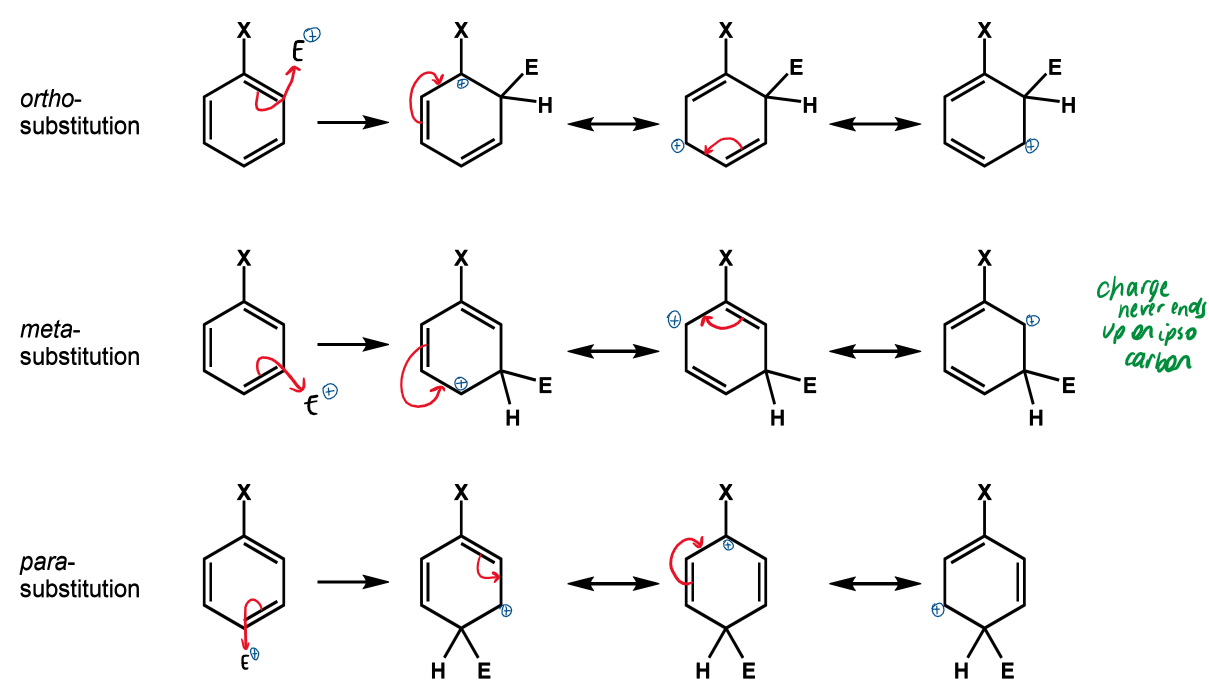
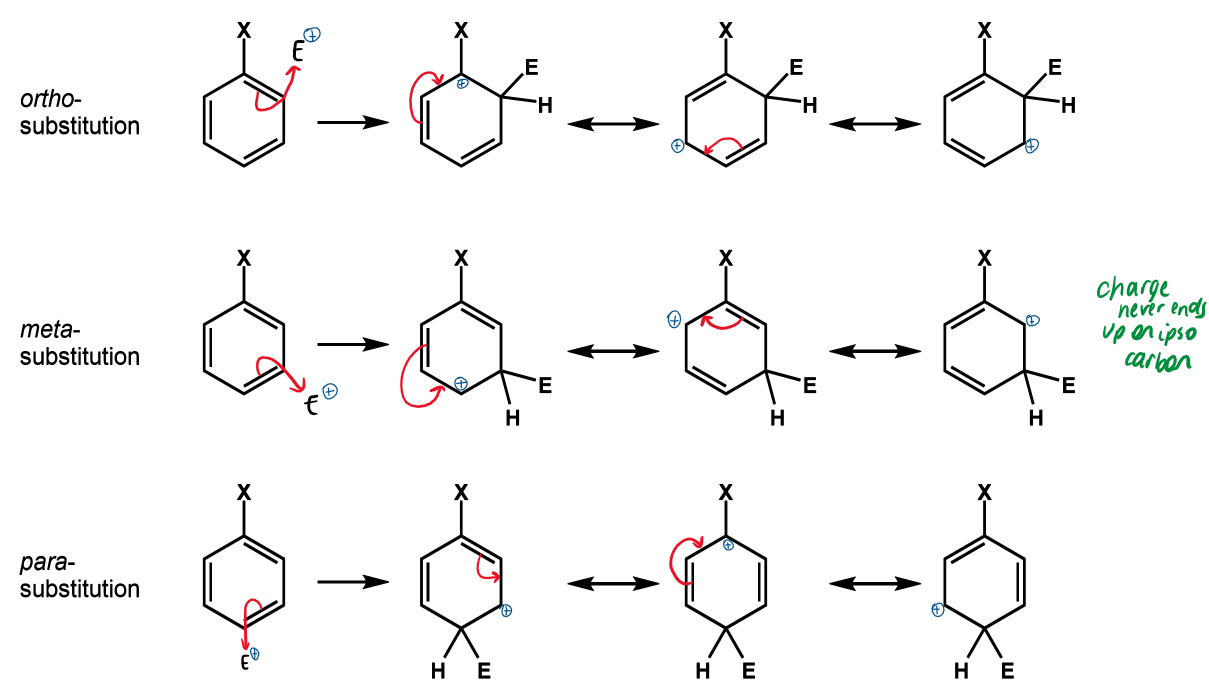
names and positions for benzene substituents
referred to as ortho, meta and para relative to substituent X
ipso is the carbon with substituent X attached
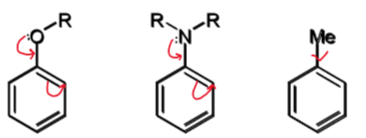
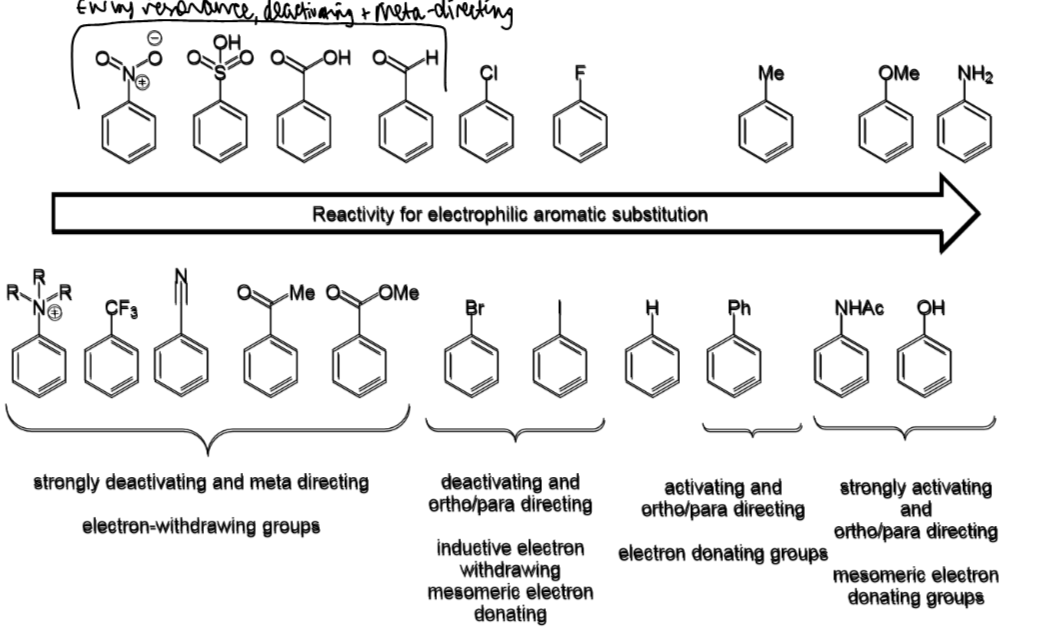
effect on SEAr if X is EDG
SEAr reactions will
be faster than for benzene
happen preferentially at ortho or para
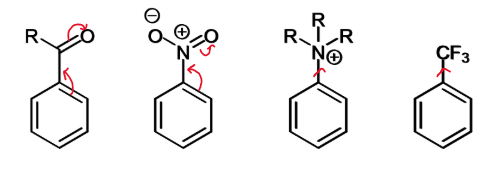
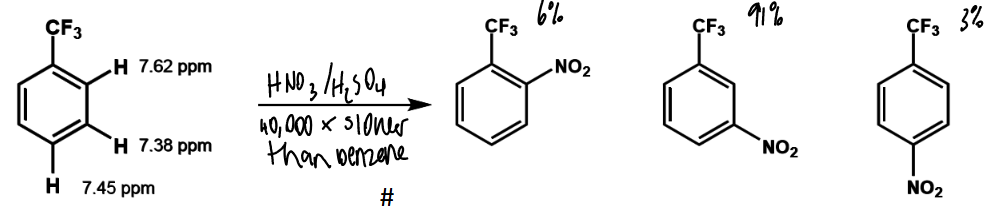
effect is X is EWG
SEAr reactions will
be slower than for benzene
happen preferentially at meta
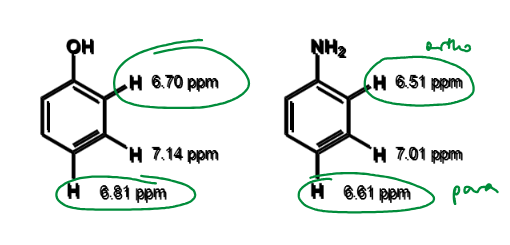
lower chemical shift = ?
more shielding
more electron density
more nucleophilic
phenol resonance forms
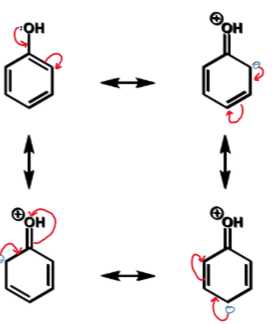
where does electrophilic attack occur
at carbon with highest electron density - chemical shift is used as a measure
effect of electron density on rate
more electron rich aromatics will react faster than benzene
when does activation by resonance occur
mesomeric electron donation
when does deactivation by resonance occur
mesomeric electron withdrawal
resonance of nitrobenzene
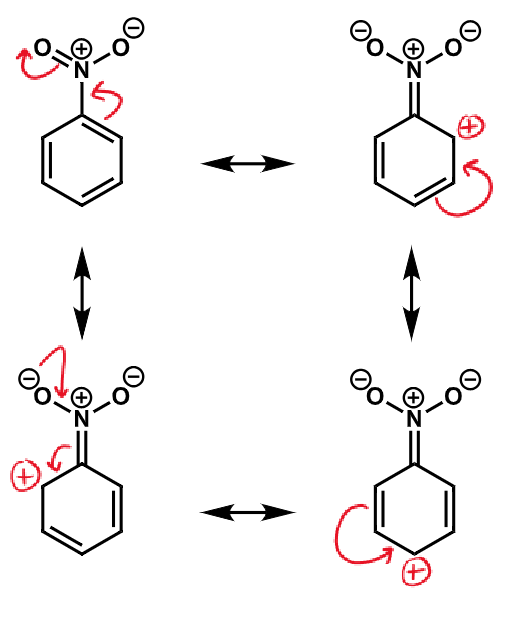
order of chemical shifts for o/m/p for activation by resonance
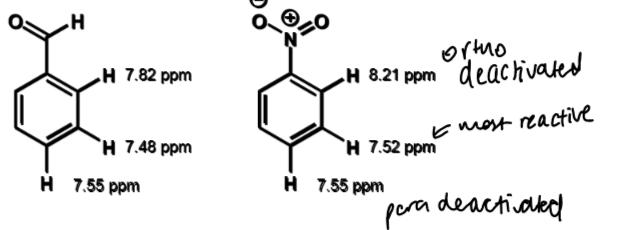
order of chemical shifts for o/m/p for deactivation by resonance
deactivated in terms of electrophilic aromatic substitution
meta is least deactivated
effect of positive charges on activation
a positive charge deactivates a position as electron density is significantly reduced and the electrophile attacks the carbon with the highest electron density
activation by induction
groups
strength
position (s)
alkyl groups cannot participate in resonance but they can activate by induction (σ donors)
weak activation
ortho/para directing
deactivation by induction
groups
strength
position(s)
EWGs eg -CF3, -NR3+ can deactivate by induction
strong deactivation
meta directing
effect of EDG on resonance
position(s) preferred
stabilise the positive charge on the ipso carbon
ortho/para substitution favoured
effect of EWG on resonance
position(s)
destabilise positive charge on ipso carbon so meta substitution favoured so that this doesn’t happen
halogen effects
deactivating by induction
ortho/para directing due to weak resonance effects
relative rates of reaction for halogen substituents
why?
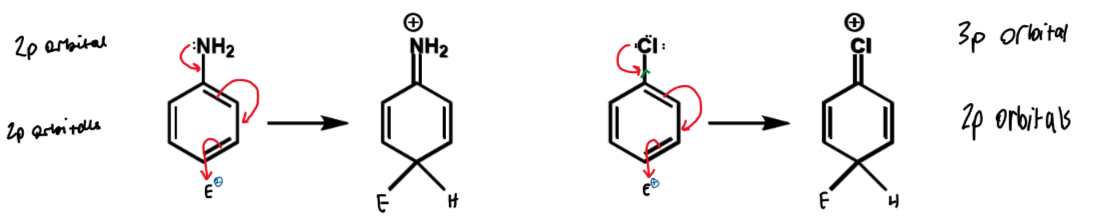
F > I > Cl > Br
due to electronegativity alone the order would be I > Br > Cl > F (as more deactivating the stronger the inductive effect) but the order is also affected by how good the resonance effects are
F’s lone pairs overlap best with the benzene pi-system due to the similar size of F and C 2p orbitals so F is more efficient at electron donation by resonance. I then has next best overlap as the 5p orbitals are not as tightly held as Cl’s 3p and Br’s 4p
N vs Cl
similar electronegativity - both should deactivate by induction
N has better orbital overlap than Cl for lone pair donation
nitrogen is activating by resonance
Cl is still o/p directing and is overall deactivating
compare the effects on reactivity (fastest → slowest) for:
EDGs
EWGs
H
halogens
mesomeric EDGs
mesomeric EDGs
EDGs
H
halogens
EWGs
what is chemoselectivity
which functional group in a molecule reacts
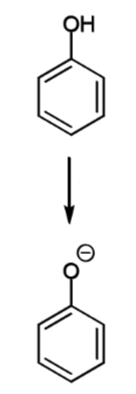
reaction of phenol
chemoselectivity
rate compared to benzene
can react at carbon or oxygen depending on the electrophile
phenol is 109 times more reactive
reaction of phenol with Br2
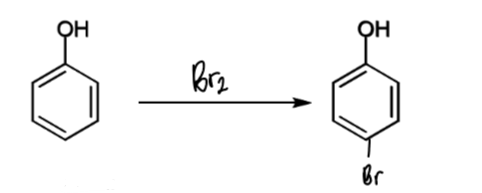
reagents/conditions?
NaOH
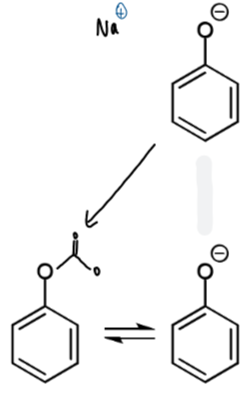
reagents/conditions for step 1
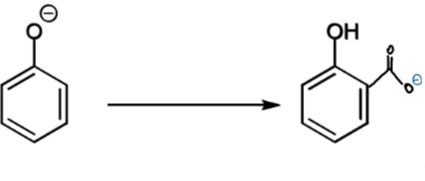
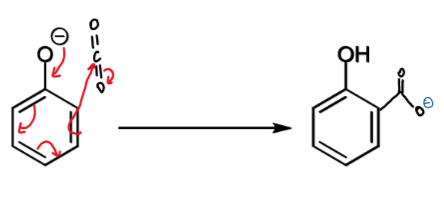
resonance for step 2
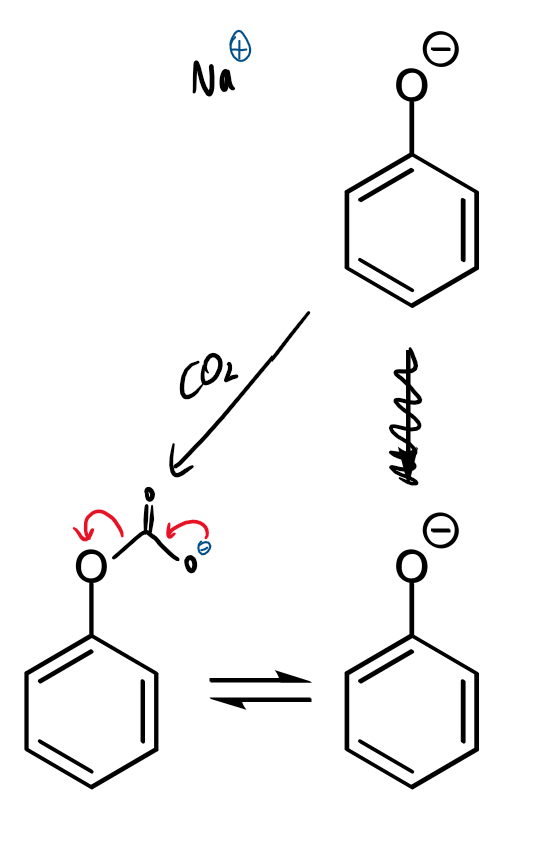
curly arrows/reagents/conditions
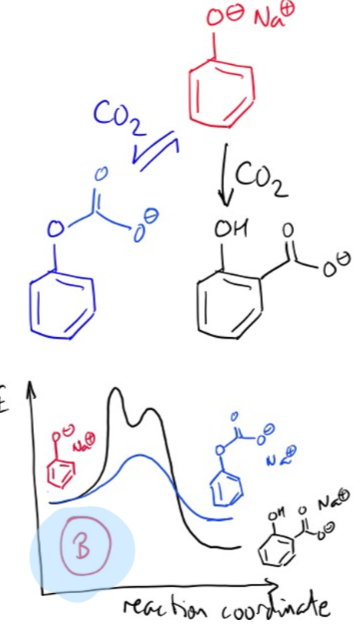
2 possibilities and rate profile for reaction of carbon dioxide with phenol
fast, reversible reaction with phenolate oxygen
slower, irreversible reaction at ortho-carbon
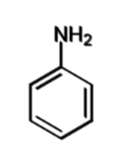
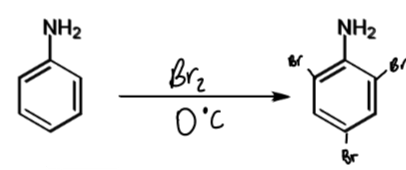
aniline
reactivity of aniline with demonstrating reaction
1014 times more reactive than benzene
forms tribromide product with benzene even in absence of Lewis acid catalyst
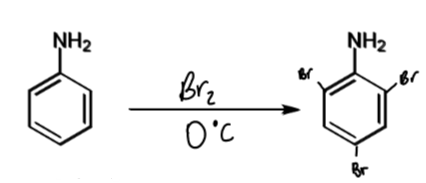
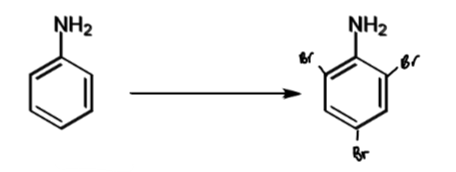
reagents/conditions
how is the reactivity of aniline controlled
why might this be done
acetylation of the amine reduces its electron donation into the aromatic ring
it also creates steric hindrance around the ortho positions
can then create a monobromide rather than tribromide
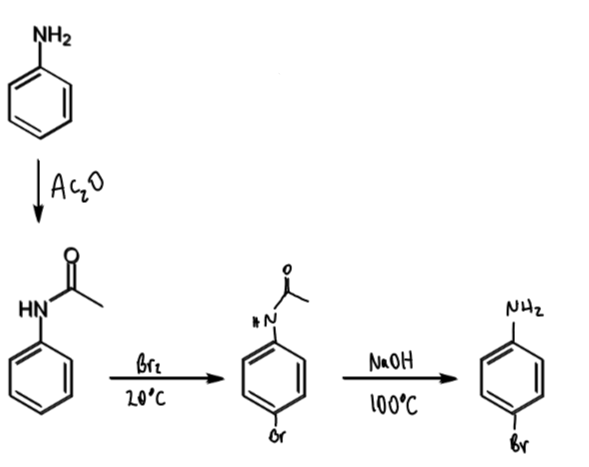
reagents/conditions
Ac2O
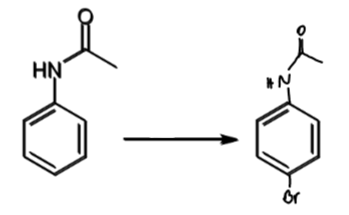
reagents/conditions
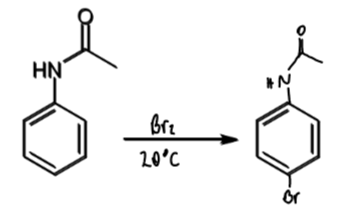
reagents/conditions
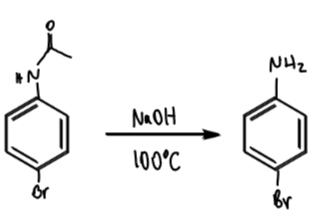
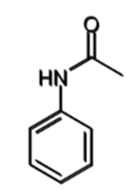
steric hindrance of acylated aniline
consequence
hinders ortho so mainly para-directing
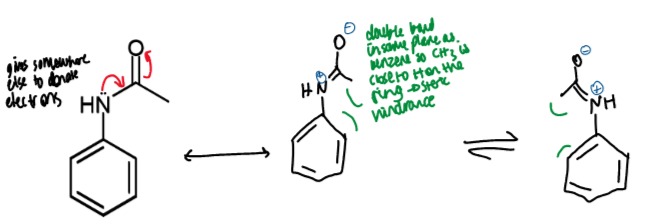
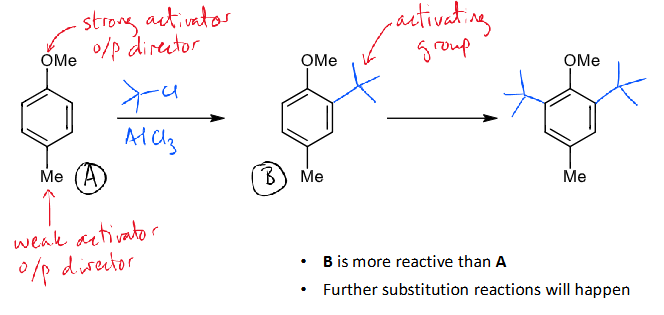
how to control number of substitutions on a benzene ring
introduce a deactivating group allows clean mono-substitution
how to stop benzene substitution at mono (what introduced and where, reagensts/conditions)
B less reactive than A
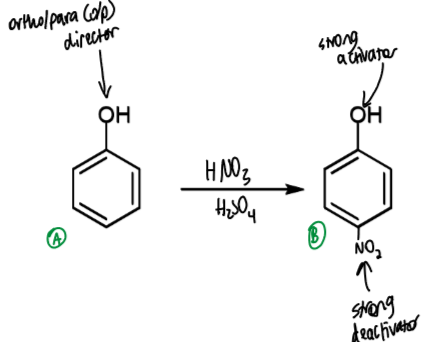
effect of activating groups on substitution
introducing an activating group encourages multiple substitutions
when there are multiple activating groups, the strongest activator determines which position reacts next
how can activating groups be introduced
mechanism + reagents/conditions
how many groups?
alkylation reactions introduce additional activating groups
further alkylations will occur until steric factors prevent additional reaction
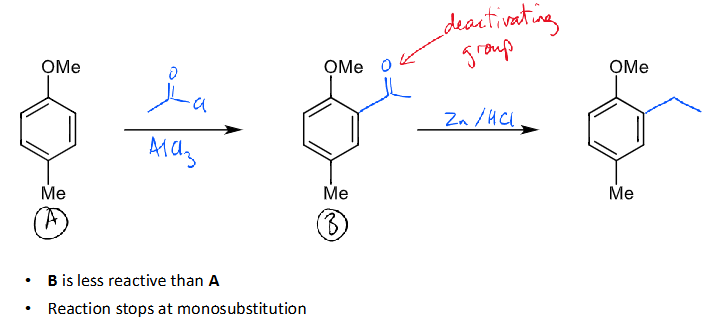
how to get monoalkylation
use friedel-crafts acylation and reduction
introducing an acyl group deactivates the ring (due to conjugation pulling electron density out of the ring), reducing the likelihood of further substitution
clemmensen reduction allows conversion to alkyl group
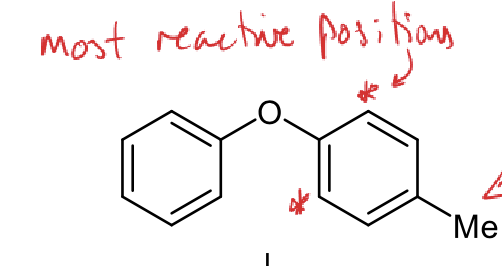
which ring is more reactive
which positions are most reactive
ring on right more reactive - extra EDG, Me weakly activating
most reactive positions are ortho positions on the right ring
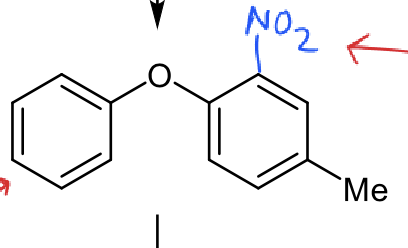
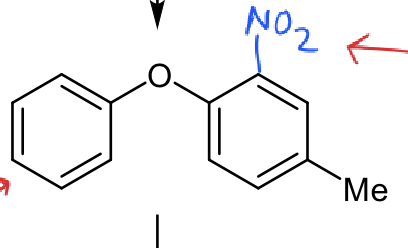
which ring is more reactive
Me weakly activating but NO2 strongly deactiviating so ring on left more reactive
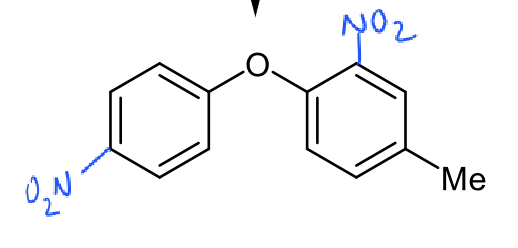
where will further nitration take place
para on left ring - least sterically hindered
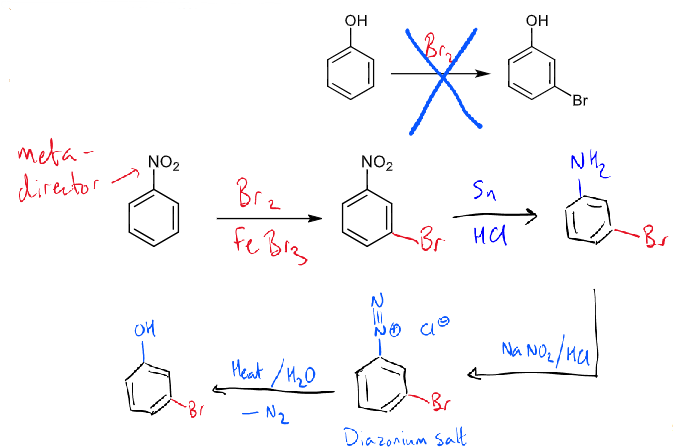
problem of meta-bromination of phenol: what the problem is and how to solve it
OH strongly o/p directing so cannot directly brominate at the meta position
solution is to use a nitro-group as a latent hydroxyl group
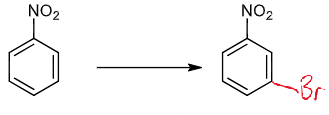
reagents/conditions
why does Br attach there
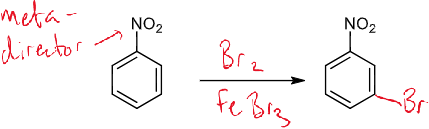

reagents/conditions
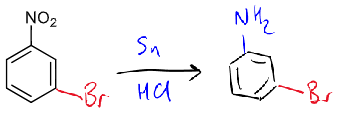
reagents/conditions
type of product
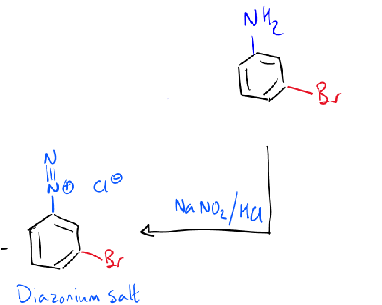
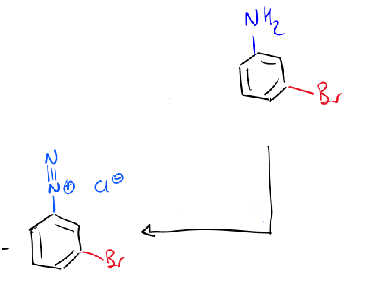
reagents/conditions
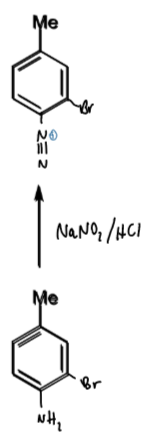
NaNO2 → diazonium ion on benzene ring
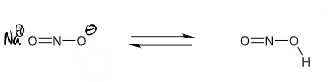
reagents/conditions
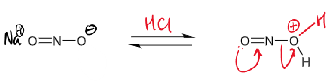
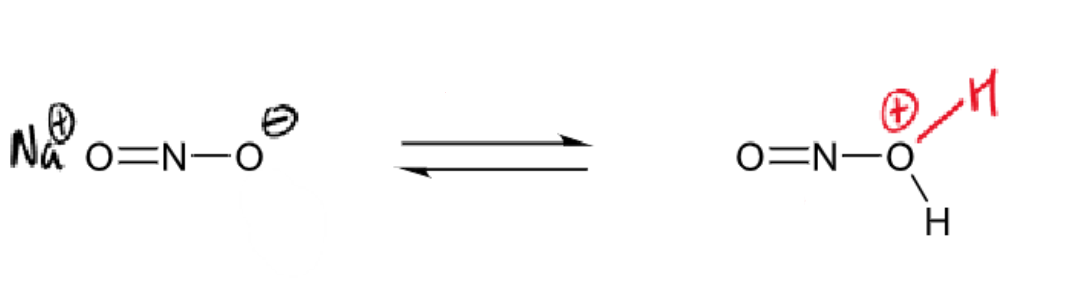
draw curly arrows/give reagents/conditions for next step
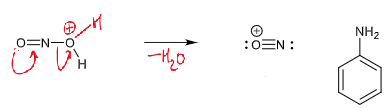
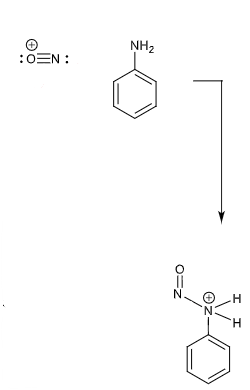
draw curly arrows
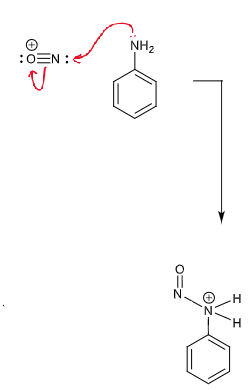
draw curly arrows
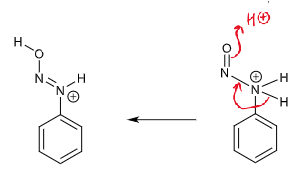
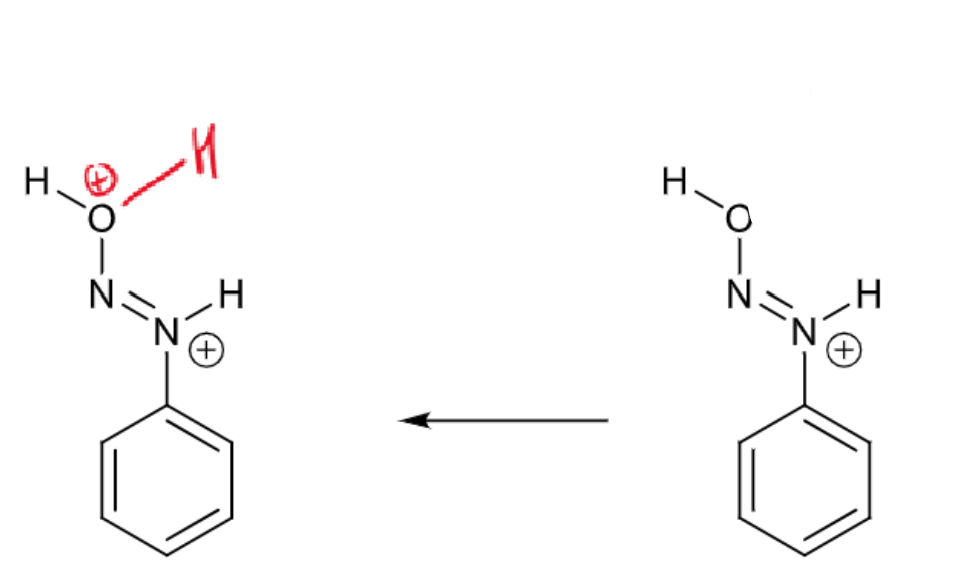
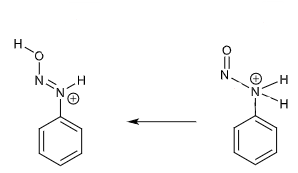
draw curly arrows
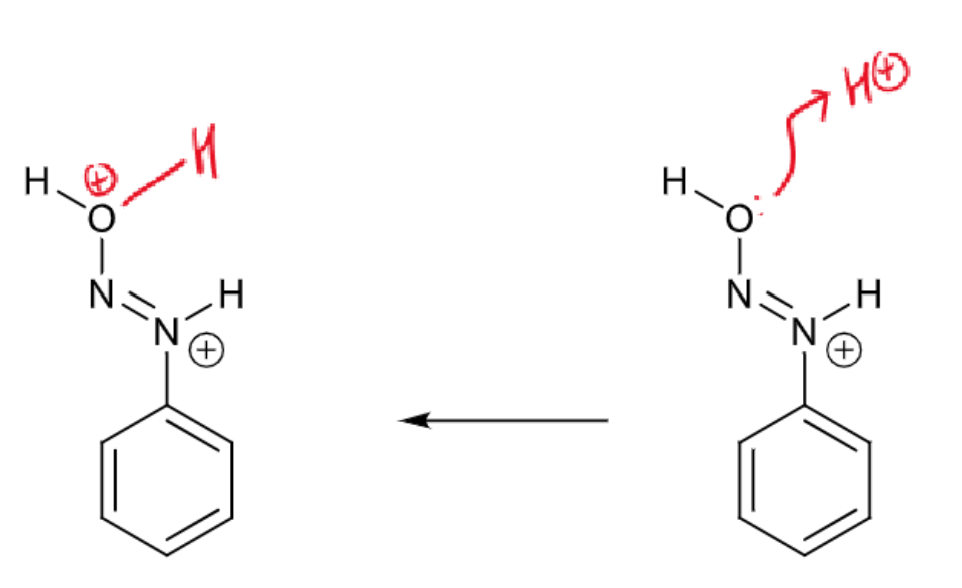
draw curly arrows
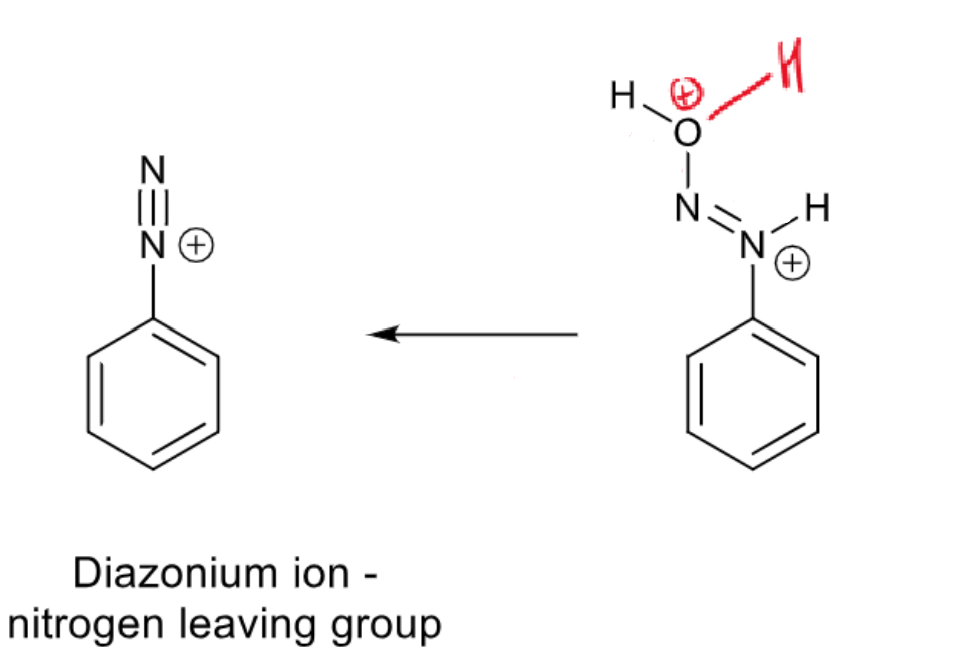
what is the product
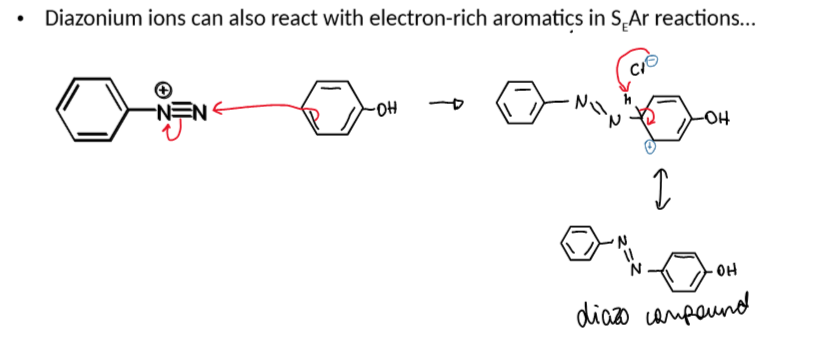
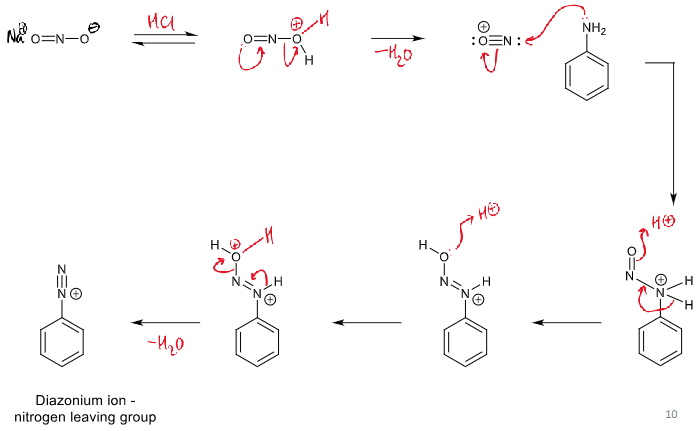
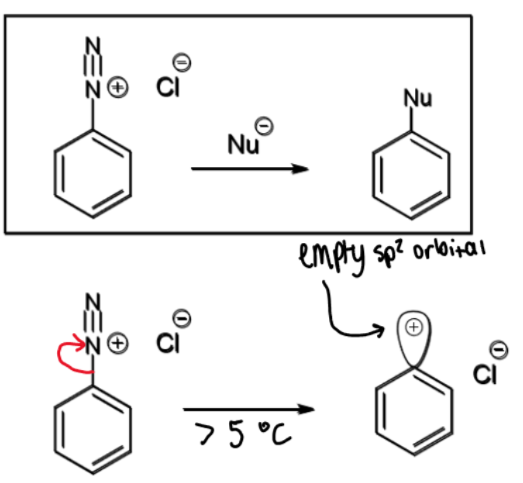
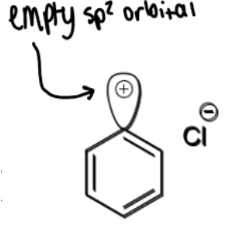
diazonium ion - nitrogen leaving group
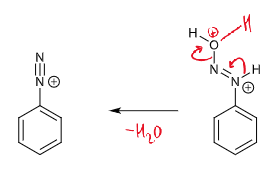
reaction of diazonium ions with Nu and conditions
at > 5ºC, N2 leaves which leaves an empty sp2 orbital which a nucleophile can attack
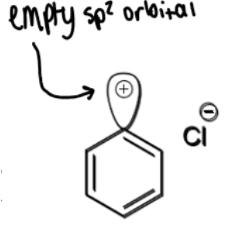
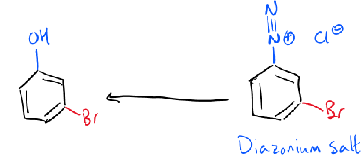
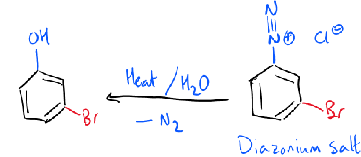
formation of phenol
+H2O
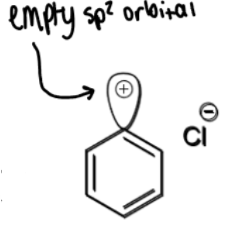
formation of iodobenzene
+KI
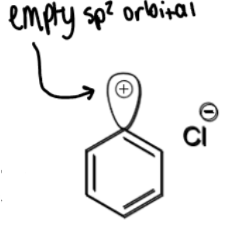
formation of X-benzene where X = Cl, Br, CN
+CuX
formation of benzene
+hypophosphorous acid
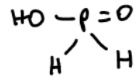
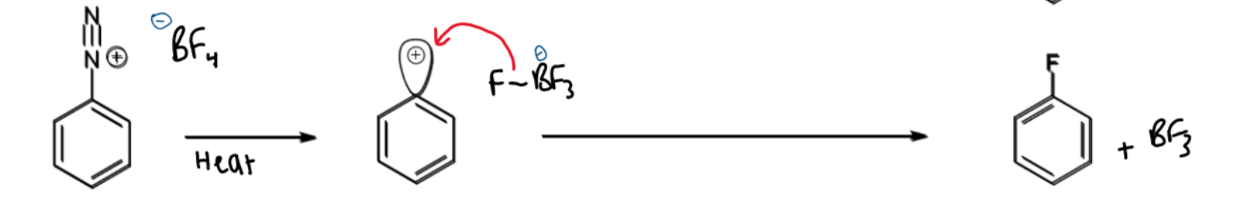
formation of fluorobenzene from diazonium ion
add HBF4 then follows mechanism
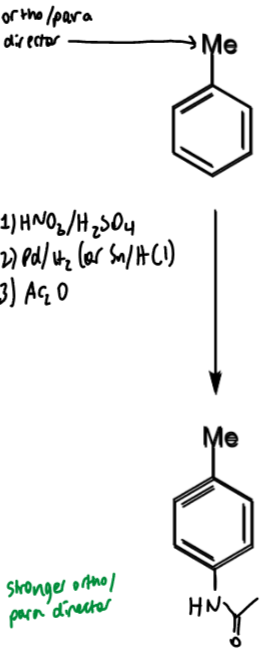
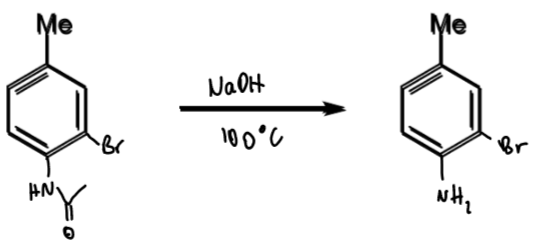
problem of meta-bromination of toluene: what is it + how to solve
Me is o/p directing so not possible to directly brominate at the meta position
solution is to temporarily introduce a stronger directing group
toluene → meta-bromotoluene
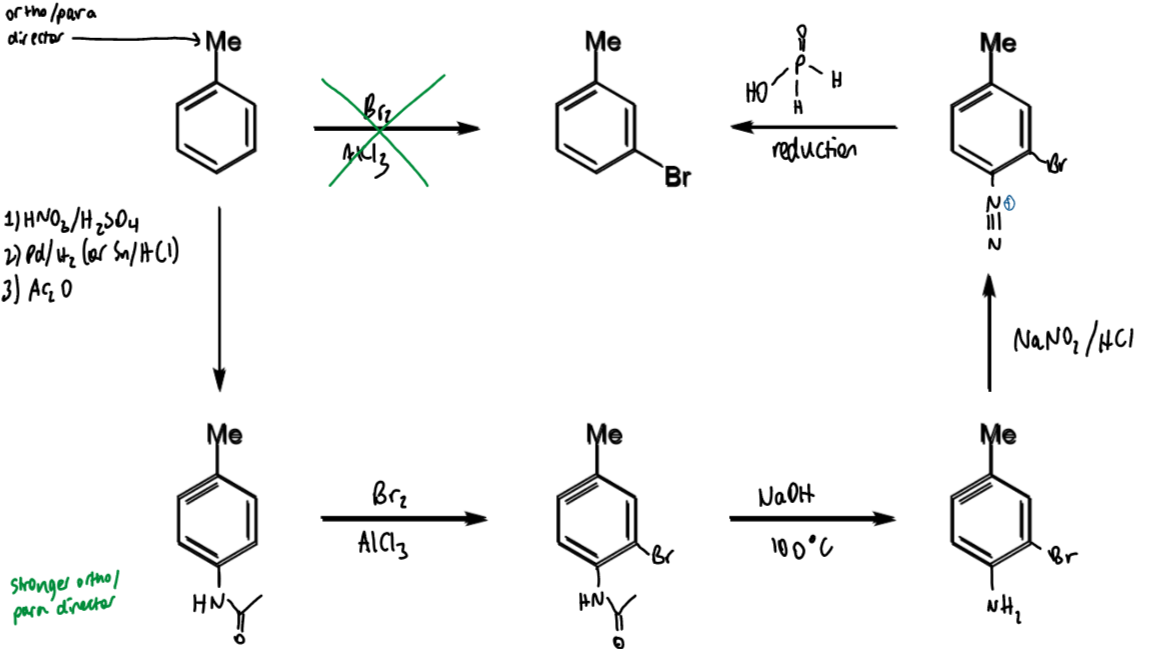
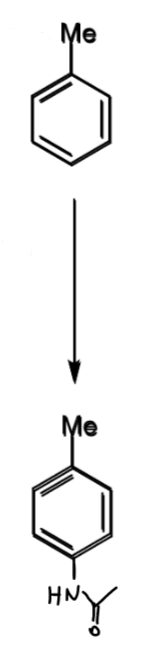
reagents/conditions
why steps done and how it helps the problem
1 - nitration
2 - amine
3 - amide
amide wanted as it is strong o/p director so can add another atom at meta to Me (as this is ortho to amide)
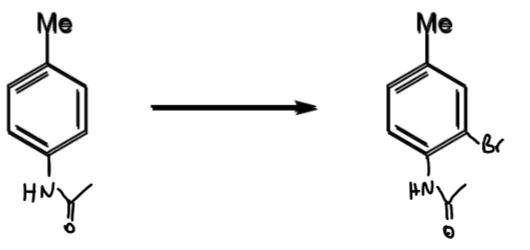
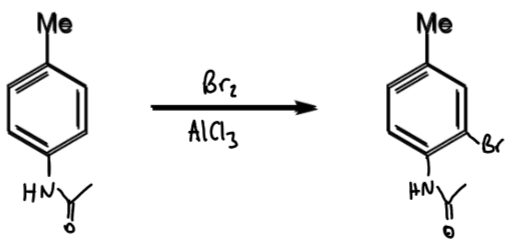
reagents/conditions
AcOH
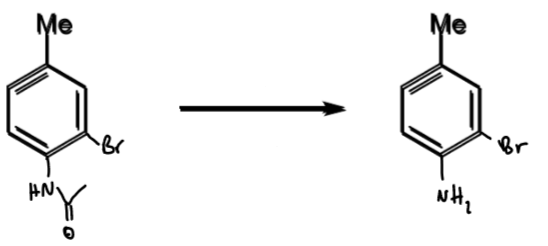
reagents/conditions
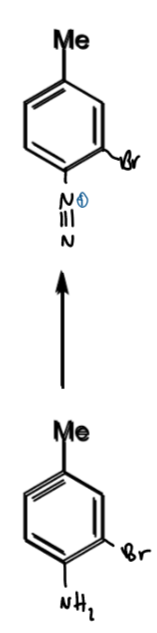
reagents/conditions
<5 degrees
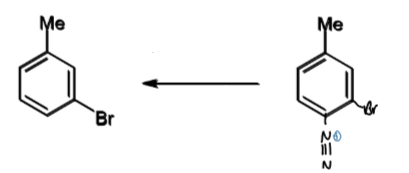
reagents/conditions
heat
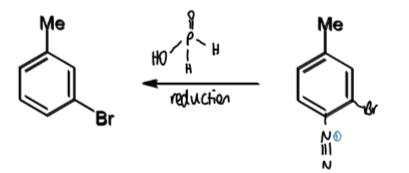
reactions of diazonium salts with nucleophiles
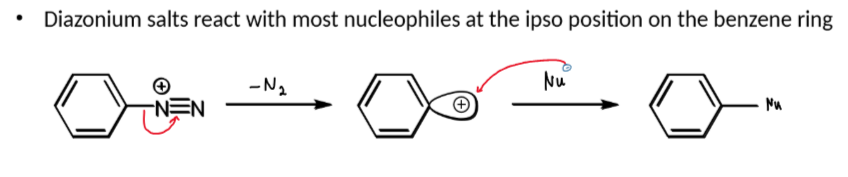
reaction of diazonium ions with phenol