f4 physics metals and metal extraction
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
physical properties of metals
· Thermal conductivity:
o Metals are generally good conductors of heat. This means they transfer thermal energy efficiently.
· Electrical conductivity:
o Metals are good conductors of electricity. This is due to the presence of delocalized electrons in their structure, which can carry an electrical charge.
· (c) Malleability and ductility:
o Malleability: Metals can be hammered or pressed into thin sheets without breaking (e.g., gold leaf).
· (d) Melting points and boiling points:
o Metals generally have high melting points and boiling points, indicating strong forces of attraction between their atoms.
physical properties of non metals
· (a) Thermal conductivity:
o Non-metals are generally poor conductors of heat (insulators).
· (b) Electrical conductivity:
poor electrical conductors (insulators) graphite is an exeption
· c) Malleability and ductility:
non metals are brittle and will shatter if hammered or stretched
· (d) Melting points and boiling points:
o Non-metals have a wider range of melting and boiling points. Some have very low melting and boiling points (e.g., gases), while others have high ones (e.g., diamond).
explain the reaction of metals with dilute acid
o Metals react with dilute acids (e.g., hydrochloric acid, sulfuric acid) to produce a salt and hydrogen gas.
o General equation: Metal + Acid → Salt + Hydrogen
Example: Magnesium + Hydrochloric acid → Magnesium chloride + Hydrogen
explain the reaction of metals with cold water and steam
o Some reactive metals (e.g., potassium, sodium, calcium) react vigorously with cold water to produce a metal hydroxide and hydrogen gas.
o Less reactive metals (e.g., magnesium) react slowly with cold water but react more quickly with steam to produce a metal oxide and hydrogen gas.
o Unreactive metals (e.g., copper, gold) do not react with water or steam.
explain the reaction of metals with oxygen
o Metals react with oxygen to form metal oxides.
o The reactivity of metals with oxygen varies. Some metals (e.g., sodium) react rapidly at room temperature, while others (e.g., iron) require heating.
o General equation: Metal + Oxygen → Metal oxide
o Example: 2Mg + O2 → 2MgO
list the uses of metals
· (a) Aluminium in aircraft manufacture:
o Low density: Aluminium is very light, which reduces the weight of the aircraft and improves fuel efficiency.
· (b) Aluminium in overhead electrical cables:
o Low density: Makes the cables lighter, reducing the need for strong supports.
o Good electrical conductivity: Allows the efficient transmission of electricity.
· (c) Aluminium in food containers:
o Resistance to corrosion: Aluminium does not react easily with air or water, protecting the food from contamination.
· (d) Copper in electrical wiring:
o Good electrical conductivity: Copper allows electricity to flow easily.
o Ductility: Copper can be drawn into thin wires.
list the reactivity series in order of most reactive to least reactive
Potassium,
sodium,
calcium,
magnesium,
aluminium,
carbon, zinc, iron, hydrogen, copper, silver, gold
describe the reaction if potassium, sodium and calcium with cold water
These metals react very vigorously with cold water, producing a metal hydroxide and hydrogen gas. The reactions are exothermic (release heat) and can be dangerous
describe the reaction of magnesium with steam
§ Magnesium reacts with steam to produce magnesium oxide and hydrogen gas. This reaction occurs more readily with steam than with cold water.
describe the reaction of magnesium, zinc, iron, copper, silver and gold with dilute hydrochloric acid
§ Magnesium, zinc, and iron react with dilute hydrochloric acid to produce a salt and hydrogen gas. The rate of reaction decreases as you go down the reactivity series.
§ Copper, silver, and gold do not react with dilute hydrochloric acid.
§ These reactions demonstrate the differing reactivities of metals. Metals higher in the reactivity series react more readily with acids.
how to deduce the order of reactivity
relate the relative reactivities of metals with positive ion formation
o The reactivity of a metal is related to its tendency to form positive ions (cations).
o More reactive metals lose electrons more easily to form positive ions.
explain what is meant by displacement reaction
o : A more reactive metal will displace a less reactive metal from its salt solution.
§ For example, zinc will displace copper from copper sulfate solution: Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s) because zinc is higher up in the reactivity series
explain the unreactivity of aluminium
o Aluminium is a reactive metal, but it appears unreactive due to the formation of a thin, strong layer of aluminium oxide (Al2O3) on its surface.
o This oxide layer is impermeable and protects the aluminium from further reaction with air, water, or dilute acids.
describe what is meant by metallic bonding
o Metallic bonding is the electrostatic attraction between positively charged metal ions and a 'sea' of delocalized electrons.
o Metal atoms are arranged in a giant metallic lattice structure.
o The outermost electrons of the metal atoms are delocalized, meaning they are not bound to any specific atom and can move freely throughout the lattice.
name the properties of metals and how they relate to their structure and bonding
o a) Good electrical conductivity:
§ The delocalized electrons are free to move and carry an electrical charge throughout the metal lattice.
o (b) Malleability and ductility:
§ The layers of positive ions in the metallic lattice can slide over each other without breaking the metallic bond.
§ The delocalized electrons maintain the bonding between the ions, even when they are moved.

describe an alloy
o An alloy is a mixture of a metal with other elements. These elements can be other metals or non-metals.
o Examples:
Brass: A mixture of copper and zinc.
Stainless steel: A mixture of iron with other elements, such as chromium, nickel, and carbon.
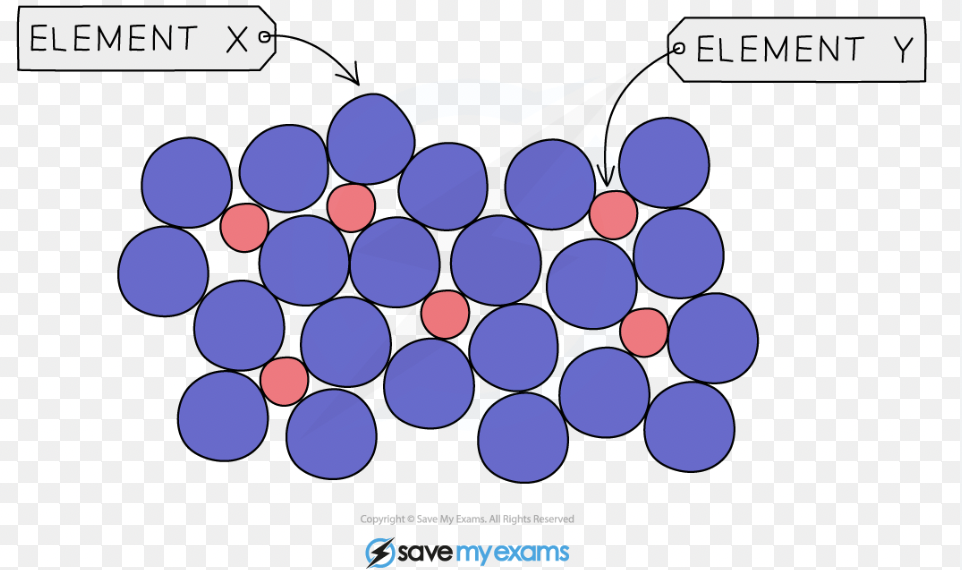
name the structure and properties of alloys
o Alloys are harder and stronger than pure metals because the different-sized atoms in the alloy disrupt the regular arrangement of the metal lattice.
This disruption prevents the layers of atoms from sliding over each other easily, making the alloy less malleable and less ductile but stronger
uses of alloys
o Alloys are often more useful than pure metals due to their enhanced properties, such as increased hardness, strength, and resistance to corrosion.
§ Alloys are used in various applications due to their specific properties:
§ Stainless steel: Used in cutlery because of its hardness and resistance to rusting (corrosion).
describe the relation of roman numerals in indicating the oxidation number of an element in a compound
§ Roman numerals are used to indicate the oxidation number of an element in a compound.
§ Example: Iron(III) oxide (Fe2O3) indicates that iron has an oxidation number of +3.
explain what is meant by redox reactions
§ Redox reactions involve both oxidation and reduction occurring simultaneously.
§ Oxidation and reduction can be defined in terms of the loss and gain of oxygen.
describe oxidation and reduction in terms of electron gain and loss
§ Oxidation is the loss of electrons.
§ Reduction is the gain of electrons.
§ In a redox reaction, one substance loses electrons (is oxidised), and another substance gains electrons (is reduced).

how to identify the oxidation and reduction of substances
§ You should be able to identify which substances are oxidised and reduced in a redox reaction by looking at the changes in electron transfer or oxygen gain/loss.

define oxidation
§ Oxidation can be defined as:
§ (a) Loss of electrons
§ (b) An increase in oxidation number
define reduction
§ Reduction can be defined as:
§ (a) Gain of electrons
§ (b) A decrease in oxidation number

describe a redox reaction in terms of electron transfer
Redox reactions are identified as reactions involving the transfer (gain and loss) of electrons
6.4.9
how to identify a redox reaction by color change
§ Redox reactions can sometimes be identified by characteristic colour changes:
§ Acidified aqueous potassium manganate(VII): Changes from purple to colourless when reduced.
§ Aqueous potassium iodide: Changes from colourless to brown when oxidised.
what is an oxidising agent?
An oxidizing agent is a substance that gains electrons in a redox reaction, causing another substance to be oxidized.
what is a reducing agent
A reducing agent is a substance that donates electrons in a redox reaction, causing another substance to be reduced.
6.4.13
what are the conditions for rusting
§ Rusting is the corrosion of iron and steel to form hydrated iron(III) oxide (rust).
§ The conditions required for rusting are:
§ Oxygen
§ Water
list common barrier methods for rust prevention
painting
greasing
covering with plastic
how do barrier methods work?
§ Barrier methods prevent rusting by excluding either oxygen or water, or both, from coming into contact with the iron or steel surface.
explain the term galvinization
galvinization is a type of sacrificial protection that involves coating iron/ steal with a layer of zinc. zinc acts as a barrier.
explain the term sacrificial protection
§ A more reactive metal (like zinc) is used to protect a less reactive metal (like iron) from corrosion.
§ The more reactive metal corrodes instead of the iron.
§ This occurs because the more reactive metal has a greater tendency to lose electrons (get oxidised) than iron.
explain how the position of a metal in the reactivity series affects its ease of extraction
describe the extraction of iron from hematite with the help of a blast furnace
§ Hematite (Fe2O3) is a common ore of iron.
§ The extraction of iron in a blast furnace involves the following steps:
§ (a) Burning of carbon (coke): Coke burns in the presence of air to produce heat and carbon dioxide: C + O2 → CO2
§ (b) Reduction of carbon dioxide: Carbon dioxide reacts with more coke to form carbon monoxide: C + CO2 → 2CO
§ (c) Reduction of iron(III) oxide: Carbon monoxide reduces iron(III) oxide to iron: Fe2O3 + 3CO → 2Fe + 3CO2
§ (d) Thermal decomposition of calcium carbonate (limestone): Limestone decomposes to produce calcium oxide and carbon dioxide: CaCO3 → CaO + CO2
§ (e) Formation of slag: Calcium oxide reacts with silica (sand) to form slag (calcium silicate): CaO + SiO2 → CaSiO3
how is aluminium extracted?
§ The main ore of aluminium is bauxite.
§ Aluminium is extracted from bauxite by electrolysis.
