Lecture #19 | Apoptosis and p53 Mutations
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Simple: p53 role in apoptosis
p53 induces programmed cell death when the DNA damage is too great to repair
p53 +/+ cells have a much lower percent survival after X-ray that p53 -/-; normal die off more after X-ray then -/-
apoptosis is induced by wildtype p53 in response to DNA damage
Where are the major players located?
Mitochondria, cytochrome c, APAF-1, Bax/Bcl2, Caspases
What are the common features in cancer?
Deregulation of cell proliferation and suppression of apoptosis
Apoptosis overview
Programmed cell death
normal developmental process
Characterized by discrete steps
Tidy verses necrosis, which is messy
changes in cell morphology
cascade of caspases
Induced by variety of internal and external signals
Intrinsic and extrinsic pathways
Role of apoptosis of normal development
Proper limb differentiation
ex: well formed digits
ex: well formed tails
immune system and mammary tissue
cell death in nervous system
Dysregulation of apoptosis is associated with neurodegenerative diseases
Discrete steps in apoptosis
Cell shrinkage
Condensation of cytoplasm
Nuclear disintegration
DNA fragmentation
Increased fragmentation as apoptosis increases
Blebbing
Apoptotic bodies
Phagocytosis
Necrosis characteristics
Messy, Unplanned cell death
cell swelling
membrane disruption
lysis of nucleus
inflammation
group of cells
messy homicide
Apoptosis characteristics
Programmed cell death
cell compaction
apoptotic body
no inflammation
surrounding cells are not affected
all evidence removed by phagocytosis
Neat, tidy suicide
Executioners of apoptosis
Called capsases
carry out killing
identify DEVD “dead box” on N-terminus for cleavage site
target of caspases:
actin in cytoskeleton → blebs
lamins → condensation of chromatin
ICAD → release CAD _ digest DNA
release Dnase 1 from actin
Regulation of caspases
Regulated by caspases
synthesized in an inactive from as procaspases
activated by protease cleavage
initiator caspases lead to the amplification of executioner caspases
Pathways to activate apoptosis
Extrinsic pathway: signal from outside
Intrinsic pathway: signal from inside
General: Extrinsic pathway
Initiator: Caspase 8
executors: Caspase 3,6,7,12
General: Intrinsic pathway
Initiator: Caspase 9
executors: Caspase 3,6,7,12
Intrinsic pathway overview
Signal: release of cytochrome c from mitochondria
Cytochrome c binds Apaf-1 (which is typically individual but becomes a flower structure), resulting in aggregation and activation of caspase 9 (initiator)
This binding forms the apoptosome= Apaf-1, cytochrome c and caspase 9
Activated caspase 9 initiates the apoptotic response by activating executioner caspases
These then cleave targets
Results in cell death
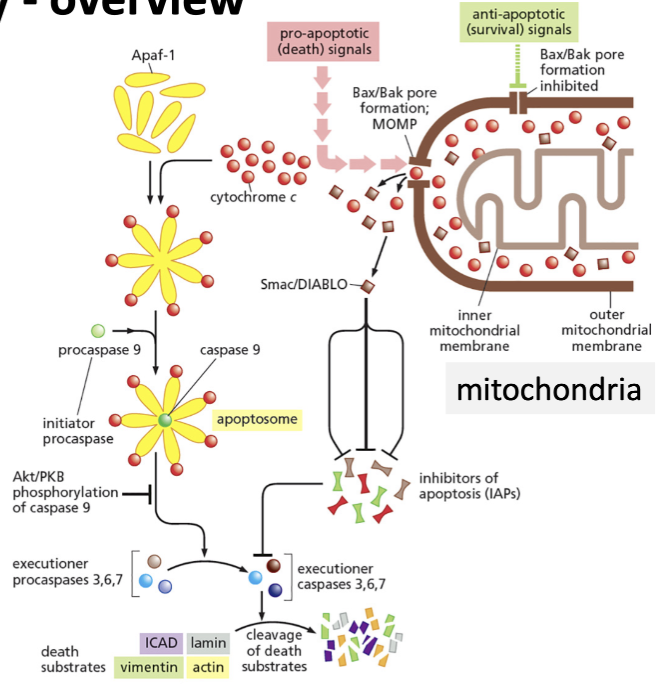
Smac/DIABLO
Inhibitors of apoptosis of executioner caspases 3,6,7
How is cytochrome c released from mitochondria?
Under stress conditions, pro-apoptotic proteins (Bad, Bim) activate Bax/Bak (other proteins founder in the outer mitochondrial membranes)
forms pores in the outer mitochondrial membrane that releases cytochrome c
open pores: release of cytochrome c
Under normal conditions, where is cytochrome c normally kept?
Anti-apoptotic proteins (Bcl) block the pores made by the Bax/ Bak proteins and sequester pro-apoptotic Bim and Bad
Bcl-2, Bax, Bak, Bim, Bad
Family of related proteins with different function
Bcl-2 has the Bh4 domain, making it pro-survival
Bax, Bak, Bim, Bad all lack BH4, making them pro-apoptotic
Since Bcl is a life signal, it must be what?
An oncogene, meaning that it will try and increase proliferation
identified as a translocation in 80% of patients with follicular b-cell lymphoma
Translocation or Bcl-2 over expression suppresses lymphocyte apoptosis
Link between Bax and Bcl and release to cytochrome from the mitochondria to cancer
p53 can upregulate Bax (tumor suppressor) and down regulate Bcl2 (oncogene)
increases Bax, increased cytochrome c
Why is p53 the most commonly mutated gene in human cancer?
p53 is the guardian of the genome
Single pt mutations can inactive p53
Mutations are dominant negative
Both alleles need to be inactivated
Mutations are seldom lethal and can actually help select for the transformed phenotype
Mutations in p53 lead to genome instability which aids cancer progression
Mutation in p53 can act at several different points in cancer progression
How can single pt mutation inactivate p53?
can prevent p53 from regulating the transcript of gene involved with guardian processes
mutations can occur at a relatively high frequency
target site is large (200 aa)
most mutations occur in the DBD (DNA binding domain) hot spots
Structure of p53 relation to dominant negative mutations?
If there is a mutation in the tetramerization domain, it will allow for dominant negative mutations
Mutations are dominant negative
Missense mutations are the most common type of mutations in p53
one amino acid is converted to a different amino acid
impacts function but not degraded
tetramerization region is typically intact and full length mutant will be formed
Mutant is not targeted by MDM2, so is available in larger concentration
What does it mean that p53 mutations are seldom lethal and can actually help select for transformed phenotype
Rarely cause death but can help select for the transformed phenotype
ex: sun burn → skin cell apoptosis
wt p53 will peel skin
mutant p53: some apoptosis but not all at same level which allows replication of mutant p53
Further UV damage will cause increase in mutation
Sp1 mutations leads to increased cancer risk
Sp1 is a transcription factor for MDM2
leads to increased MDM2
decreased p53