3.2.1 - Enthalpy Changes -
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
a) What energy change is making bonds associated with?
Energy is released to make bonds - exothermic bonds
What energy change is breaking bonds associated with?
Energy is taken in to break bonds - endothermic reaction
b-c) Draw enthalpy diagram?
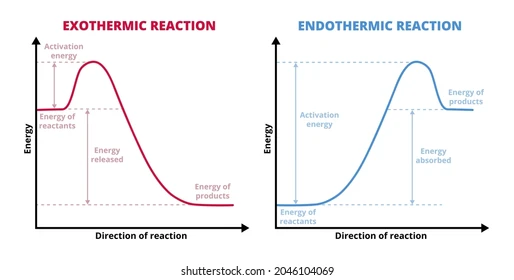
di) What are the standard conditions?
298K
100 kPa
What is activation energy?
The minimum energy required for a reaction take place
di) Define enthalpy change of reaction?
The energy change associated with given reaction
dii) Define enthalpy change of formation?
The energy change that takes place when 1 mole of a compound is formed for its reactants under state conditions
diii) Define enthalpy change of combustion?
The energy change that takes place when 1 mole of a substance is completely combusted
dv) Define enthalpy change of neutralisation?
The energy change that takes place when 1 mole of water is formed from a neutralisation reaction
e) How can you calculate enthalpy change from experimental data?
Q= mcT
Q = energy
M = mass
C = Specific hear capacity = 4.18gJ-1K-1
T = temperature
What are the advantages of using a calorimeter?
Minimises heat loss
Pure oxygen used → ensures combustion
Why might experimental methods for enthalpy determination may not be accurate?
Heat is lost to the surroundings
Not in standard conditions
Reaction may not go into completion
How to calculate enthalpy change of reaction using energy bonds?
Bond energy reactants - Bond energy of products